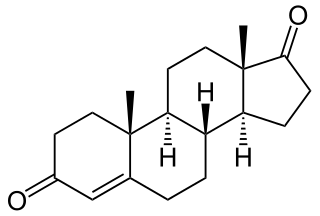
Androstenedione, or 4-androstenedione, also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol).
7alpha-hydroxycholest-4-en-3-one 12alpha-hydroxylase (EC 1.14.14.139, previously EC 1.14.13.95) is an enzyme that catalyzes the chemical reaction:
In enzymology, an alkanesulfonate monooxygenase (EC 1.14.14.5) is an enzyme that catalyzes the chemical reaction
In enzymology, an androst-4-ene-3,17-dione monooxygenase (EC 1.14.99.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3,4-dihydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione 4,5-dioxygenase (EC 1.13.11.25) is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
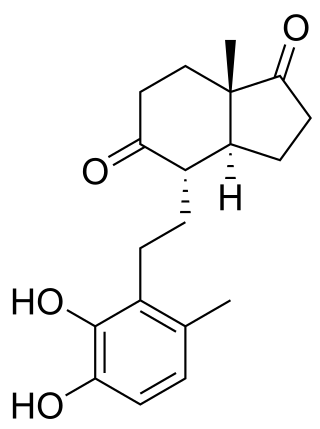
3,4-DHSA is an organic compound which is the intermediate product of the metabolism of cholesterol, by the bacteria most commonly responsible for tuberculosis. 3,4-DHSA is an acronym for 3,4-dihydroxy-9,10-seco-androst-1,3,5(10)-triene-9,17-dione, the official name of this substance. It is classified as a secosteroid, since one of the four rings of cholesterol from which it is derived is broken.
Methylsterol monooxygenase (EC 1.14.13.72, methylsterol hydroxylase, 4-methylsterol oxidase, 4,4-dimethyl-5alpha-cholest-7-en-3beta-ol,hydrogen-donor:oxygen oxidoreductase (hydroxylating)) is an enzyme with systematic name 4,4-dimethyl-5alpha-cholest-7-en-3beta-ol,NAD(P)H:oxygen oxidoreductase (hydroxylating). This enzyme catalyses the following chemical reaction
Valine N-monooxygenase (EC 1.14.13.118, CYP79D1, CYP79D2) is an enzyme with systematic name L-valine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Phenylalanine N-monooxygenase (EC 1.14.14.40, phenylalanine N-hydroxylase, CYP79A2) is an enzyme with systematic name L-phenylalanine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
3-hydroxyindolin-2-one monooxygenase (EC 1.14.13.139, BX4 (gene), CYP71C1 (gene)) is an enzyme with systematic name 3-hydroxyindolin-2-one,NAD(P)H:oxygen oxidoreductase (2-hydroxy-2H-1,4-benzoxazin-3(4H)-one-forming). This enzyme catalyses the following chemical reaction
2-Hydroxy-1,4-benzoxazin-3-one monooxygenase (EC 1.14.13.140, BX5 (gene), CYP71C3 (gene)) is an enzyme with systematic name 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one,NAD(P)H:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
3-ketosteroid 9alpha-monooxygenase (EC 1.14.13.142, KshAB, 3-ketosteroid 9alpha-hydroxylase) is an enzyme with systematic name androsta-1,4-diene-3,17-dione,NADH:oxygen oxidoreductase (9alpha-hydroxylating). This enzyme catalyses the following chemical reaction
Nitrilotriacetate monooxygenase (EC 1.14.14.10) is an enzyme with systematic name nitrilotriacetate,FMNH2:oxygen oxidoreductase (glyoxylate-forming). This enzyme catalyses the following chemical reaction
4,5:9,10-diseco-3-hydroxy-5,9,17-trioxoandrosta-1(10),2-diene-4-oate hydrolase (EC 3.7.1.17, tesD (gene), hsaD (gene)) is an enzyme with systematic name 4,5:9,10-diseco-3-hydroxy-5,9,17-trioxoandrosta-1(10),2-diene-4-oate hydrolase ( (2Z,4Z)-2-hydroxyhexa-2,4-dienoate-forming). This enzyme catalyses the following chemical reaction

16β,17α-Epiestriol, or 16,17-epiestriol, also known as 16β-hydroxy-17α-estradiol, as well as estra-1,3,5(10)-triene-3,16β,17α-triol, is a minor and weak endogenous steroidal estrogen that is related to 17α-estradiol and estriol. Along with estriol, 16β,17α-epiestriol has been detected in the urine of women during the late pregnancy stage. It shows preferential affinity for the ERβ over the ERα.

20α-Dihydrotrengestone (20α-DHTG), also known as 20α-hydroxytrengestone, as well as 6-chloro-20(S)-hydroxy-9β,10α-pregna-1,4,6-trien-3-one, is a progestin and the major active metabolite of trengestone. It appears that trengestone is a prodrug of 20α-DHTG, as it is largely transformed into this metabolite when given orally in humans. 20α-DHTG has potent progestogenic activity similarly to trengestone.

Estriol sulfamate, or estriol 3-O-sulfamate, is a synthetic estrogen and estrogen ester which was never marketed. It is the C3 sulfamate ester of estriol. The drug shows substantially improved oral estrogenic potency relative to estriol in rats but without an increase in hepatic estrogenic potency. However, the closely related compound estradiol sulfamate (E2MATE) failed to show estrogenic activity in humans, which is due to the fact that it is additionally a highly potent inhibitor of steroid sulfatase which regulates the estrogenicity of such compounds and thus it prevents its own bioactivation into estradiol.






