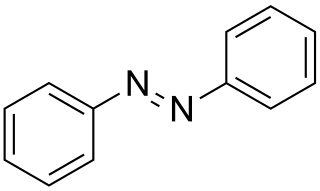
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

In chemistry, pi stacking refers to the presumptive attractive, noncovalent pi interactions between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings is electrostatically repulsive. What is more commonly observed is either a staggered stacking or pi-teeing interaction both of which are electrostatic attractive For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties. Fluorene is mildly acidic.
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
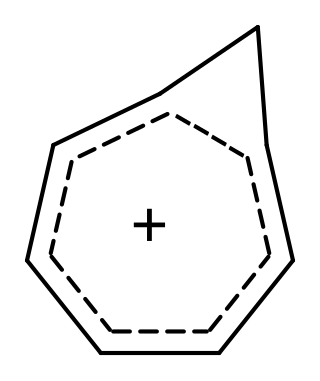
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.
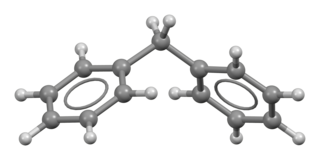
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.
F number is a correlation number used in the analysis of polycyclic aromatic hydrocarbons (PAHs) as a descriptor of their hydrophobicity and molecular size. It was proposed by Robert Hurtubise and co-workers in 1977.

Carbon nanotube chemistry involves chemical reactions, which are used to modify the properties of carbon nanotubes (CNTs). CNTs can be functionalized to attain desired properties that can be used in a wide variety of applications. The two main methods of CNT functionalization are covalent and non-covalent modifications.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.
In chemistry, vinylidenes are compounds with the functional group C=CH2. An example is 1,1-dichloroethene (CCl2=CH2) commonly called vinylidene chloride. It and vinylidene fluoride are precursors to commercially useful polymers.

9-Fluorenylidene is an aryl carbene derived from the bridging methylene group of fluorene. Fluorenylidene has the unusual property that the triplet ground state is only 1.1 kcal/mol lower in energy than the singlet state. For this reason, fluorenylidene has been studied extensively in organic chemistry.

1,1-Diphenylethylene is an aromatic hydrocarbon with chemical formula C14H12.
Catalyst transfer polymerization (CTP), or catalyst transfer polycondensation, is a type of living chain-growth polymerization that is used for synthesizing conjugated polymers. Benefits to using CTP over other methods are low polydispersity and control over number average molecular weight in the resulting polymers. Very few monomers have been demonstrated to undergo CTP.

An indenofluorene (IF) is any of five hydrocarbons with formula C
20H
12, whose carbon skeleton is a sequence of five fused rings with 6, 5, 6, 5, and 6 carbon atoms; an arrangement that can be described as the fusion of an indene core and a fluorene core.

Rajendra Rathore was an organic chemist and professor at Marquette University in Milwaukee, Wisconsin as Pfletschinger-Habermann professor of organic chemistry. He made important contributions in the area of supramolecular chemistry, synthesis of novel electro-active molecules, and drug discovery. Rathore died on February 16, 2018, after complications from chronic pulmonary sarcoidosis.
Yoshio Okamoto is a Japanese chemist, who was awarded the 2019 Japan Prize for his groundbreaking work in asymmetric polymerization and its practical applications in drug discovery.
Francis S. Mair is a British chemist and a Senior Lecturer in the Department of Chemistry at The University of Manchester. His research is based on synthetic chemistry, inorganic chemistry, catalysis and polymer chemistry.












