Eumycetoma, also known as Madura foot, is a persistent fungal infection of the skin and the tissues just under the skin, affecting most commonly the feet, although it can occur in hands and other body parts. It starts as a painless wet nodule, which may be present for years before ulceration, swelling, grainy discharge and weeping from sinuses and fistulae, followed by bone deformity.
Fungal keratitis is a fungal infection of the cornea, which can lead to blindness. It generally presents with a red, painful eye and blurred vision. There is also increased sensitivity to light, and excessive tears or discharge.

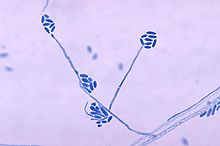
Sporothrix schenckii, a fungus that can be found worldwide in the environment, is named for medical student Benjamin Schenck, who in 1896 was the first to isolate it from a human specimen. The species is present in soil as well as in and on living and decomposing plant material such as peat moss. It can infect humans as well as animals and is the causative agent of sporotrichosis, commonly known as "rose handler's disease." The most common route of infection is the introduction of spores to the body through a cut or puncture wound in the skin. Infection commonly occurs in otherwise healthy individuals but is rarely life-threatening and can be treated with antifungals. In the environment it is found growing as filamentous hyphae. In host tissue it is found as a yeast. The transition between the hyphal and yeast forms is temperature dependent making S. schenckii a thermally dimorphic fungus.

Epichloë is a genus of ascomycete fungi forming an endophytic symbiosis with grasses. Grass choke disease is a symptom in grasses induced by some Epichloë species, which form spore-bearing mats (stromata) on tillers and suppress the development of their host plant's inflorescence. For most of their life cycle however, Epichloë grow in the intercellular space of stems, leaves, inflorescences, and seeds of the grass plant without incurring symptoms of disease. In fact, they provide several benefits to their host, including the production of different herbivore-deterring alkaloids, increased stress resistance, and growth promotion.

Acremonium is a genus of fungi in the family Hypocreaceae. It used to be known as Cephalosporium.

Cochliobolus lunatus is a fungal plant pathogen that can cause disease in humans and other animals. The anamorph of this fungus is known as Curvularia lunata, while C. lunatus denotes the teleomorph or sexual stage. They are, however, the same biological entity. C. lunatus is the most commonly reported species in clinical cases of reported Cochliobolus infection.

Setosphaeria rostrata is a heat tolerant fungus with an asexual reproductive form (anamorph) known as Exserohilum rostratum. This fungus is a common plant pathogen, causing leaf spots as well as crown rot and root rot in grasses. It is also found in soils and on textiles in subtropical and tropical regions. Exserohilum rostratum is one of the 35 Exserohilum species implicated uncommonly as opportunistic pathogens of humans where it is an etiologic agent of sinusitis, keratitis, skin lesions and an often fatal meningoencephalitis. Infections caused by this species are most often seen in regions with hot climates like Israel, India and the southern USA.
Microdochium panattonianum is a fungal plant pathogen. This pathogen causes anthracnose of lettuce, a disease which produces necrotic lesions in cultivated lettuce. In extended periods of wet weather, M. panattonianum can cause significant crop-losses. The impact of this pathogen is exacerbated by farming lettuce without crop rotation, and by planting of susceptible lettuce varieties, such as Romaine lettuce.

Fusarium solani is a species complex of at least 26 closely related filamentous fungi in the division Ascomycota, family Nectriaceae. It is the anamorph of Nectria haematococca. It is a common soil fungus and colonist of plant materials. Fusarium solani is implicated in plant diseases as well as in serious human diseases such as fungal keratitis.

Helminthosporium solani is a fungal plant pathogen responsible for the plant disease known as silver scurf. Silver scurf is a blemish disease, meaning the effect it has on tubers is mostly cosmetic and affects "fresh market, processing and seed tuber potatoes." There are some reports of it affecting development, meaning growth and tuber yield. This is caused by light brown lesions, which in turn change the permeability of tuber skin and then it causes tuber shrinkage and water loss, which finally causes weight loss. The disease has become economically important because silver scurf affected potatoes for processing and direct consumption have been rejected by the industry. The disease cycle can be divided into two stages: field and storage. It is mainly a seed borne disease and the primary source of inoculum is mainly infected potato seed tubers. Symptoms develop and worsen in storage because the conditions are conducive to sporulation. The ideal conditions for the spread of this disease are high temperatures and high humidity. There are also many cultural practices that favor spread and development. There are multiple ways to help control the disease.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

Piedraia hortae is a superficial fungus that exists in the soils of tropical and subtropical environments and affects both sexes of all ages. The fungus grows very slowly, forming dark hyphae, which contain chlamydoconidia cells and black colonies when grown on agar. Piedraia hortae is a dermatophyte and causes a superficial fungal infection known as black piedra, which causes the formation of black nodules on the hair shaft and leads to progressive weakening of the hair. The infection usually infects hairs on the scalp and beard, but other varieties tend to grow on pubic hairs. The infection is usually treated with cutting or shaving of the hair and followed by the application of anti-fungal and topical agents. The fungus is used for cosmetic purposes to darken hair in some societies as a symbol of attractiveness.
Sagenomella is a genus of filamentous Ascomycota fungus that has reported to cause systemic illness in animals. The genus was circumscribed by Walter Gams in 1978.

Apophysomyces variabilis is an emerging fungal pathogen that can cause serious and sometimes fatal infection in humans. This fungus is a soil-dwelling saprobe with tropical to subtropical distribution. It is a zygomycete that causes mucormycosis, an infection in humans brought about by fungi in the order Mucorales. Infectious cases have been reported globally in locations including the Americas, Southeast Asia, India, and Australia. Apophysomyces variabilis infections are not transmissible from person to person.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.
Scedosporiosis is the general name for any mycosis – i.e., fungal infection – caused by a fungus from the genus Scedosporium. Current population-based studies suggest Scedosporium prolificans and Scedosporium apiospermum to be among the most common infecting agents from the genus, although infections caused by other members thereof are not unheard of. The latter is an asexual form (anamorph) of another fungus, Pseudallescheria boydii. The former is a "black yeast", currently not characterized as well, although both of them have been described as saprophytes.

Neoscytalidium dimidiatum was first described in 1933 as Hendersonula toruloidea from diseased orchard trees in Egypt. Decades later, it was determined to be a causative agent of human dermatomycosis-like infections and foot infections predominantly in tropical areas; however the fungus is considered to be widespread. A newer name, Scytalidium dimidiatum, was applied to a synanamorph of Nattrassia mangiferae, otherwise known as Neofusicoccum mangiferae. Substantial confusion has arisen in the literature on this fungus resulting from the use of multiple different names including Torula dimidiata, Fusicoccum dimidiatum, Scytalidium dimidiatum, and Hendersonula toruloidea. Additionally, Scytalidium lignicola and Scytalidium lignicolum are often considered earlier names of N. dimidiatum.

Sagenomella keratitidis is a hyphomycete discovered as its own species in 2008 by Sung-Yaon Hsieh et al. at the Institute of Plant and Microbial Biology and the National Taiwan University Hospital.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Myriodontium keratinophilum is a fungus widespread in nature, most abundantly found in keratin-rich environments such as feathers, nails and hair. Despite its ability to colonize keratinous surfaces of human body, the species has been known to be non-pathogenic in man and is phylogentically distant to other human pathogenic species, such as anthropophilic dermatophytes. However, its occasional isolation from clinical specimens along with its keratinolytic properties suggest the possibility it may contribute to disease.