A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.
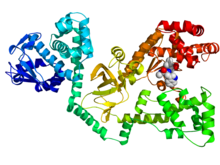
Pyruvate dehydrogenase lipoamide kinase isozyme 1, mitochondrial is an enzyme that in humans is encoded by the PDK1 gene. It codes for an isozyme of pyruvate dehydrogenase kinase (PDK).
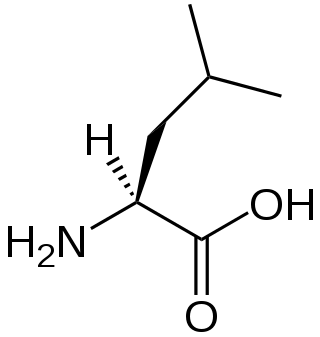
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.
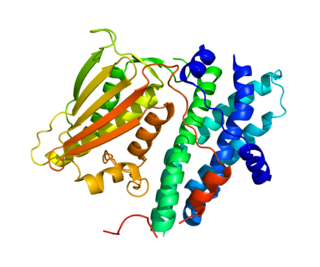
GTPase HRas, from "Harvey Rat sarcoma virus", also known as transforming protein p21 is an enzyme that in humans is encoded by the HRAS gene. The HRAS gene is located on the short (p) arm of chromosome 11 at position 15.5, from base pair 522,241 to base pair 525,549. HRas is a small G protein in the Ras subfamily of the Ras superfamily of small GTPases. Once bound to Guanosine triphosphate, H-Ras will activate a Raf kinase like c-Raf, the next step in the MAPK/ERK pathway.
Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

Furin is a protease, a proteolytic enzyme that in humans and other animals is encoded by the FURIN gene. Some proteins are inactive when they are first synthesized, and must have sections removed in order to become active. Furin cleaves these sections and activates the proteins. It was named furin because it was in the upstream region of an oncogene known as FES. The gene was known as FUR and therefore the protein was named furin. Furin is also known as PACE. A member of family S8, furin is a subtilisin-like peptidase.
Receptor activator of nuclear factor κ B (RANK), also known as TRANCE receptor or TNFRSF11A, is a member of the tumor necrosis factor receptor (TNFR) molecular sub-family. RANK is the receptor for RANK-Ligand (RANKL) and part of the RANK/RANKL/OPG signaling pathway that regulates osteoclast differentiation and activation. It is associated with bone remodeling and repair, immune cell function, lymph node development, thermal regulation, and mammary gland development. Osteoprotegerin (OPG) is a decoy receptor for RANKL, and regulates the stimulation of the RANK signaling pathway by competing for RANKL. The cytoplasmic domain of RANK binds TRAFs 1, 2, 3, 5, and 6 which transmit signals to downstream targets such as NF-κB and JNK.
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.
A protein kinase inhibitor (PKI) is a type of enzyme inhibitor that blocks the action of one or more protein kinases. Protein kinases are enzymes that phosphorylate (add a phosphate, or PO4, group) to a protein and can modulate its function.
In enzymology, a pyrroloquinoline-quinone synthase (EC 1.3.3.11) is an enzyme that catalyzes the chemical reaction

Dual specificity mitogen-activated protein kinase kinase 2 is an enzyme that in humans is encoded by the MAP2K2 gene. It is more commonly known as MEK2, but has many alternative names including CFC4, MKK2, MAPKK2 and PRKMK2.

POU domain, class 2, transcription factor 1 is a protein that in humans is encoded by the POU2F1 gene.
Dynein light chain 1, cytoplasmic is a protein that in humans is encoded by the DYNLL1 gene.

Dual specificity mitogen-activated protein kinase kinase 7, also known as MAP kinase kinase 7 or MKK7, is an enzyme that in humans is encoded by the MAP2K7 gene. This protein is a member of the mitogen-activated protein kinase kinase family. The MKK7 protein exists as six different isoforms with three possible N-termini and two possible C-termini.

Mitogen-activated protein kinase kinase kinase 7-interacting protein 1 is an enzyme that in humans is encoded by the TAB1 gene.

Eukaryotic translation initiation factor 2-alpha kinase 3, also known as protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), is an enzyme that in humans is encoded by the EIF2AK3 gene.

NLRP1 encodes NACHT, LRR, FIIND, CARD domain and PYD domains-containing protein 1 in humans. NLRP1 was the first protein shown to form an inflammasome. NLRP1 is expressed by a variety of cell types, which are predominantly epithelial or hematopoietic. The expression is also seen within glandular epithelial structures including the lining of the small intestine, stomach, airway epithelia and in hairless or glabrous skin. NLRP1 polymorphisms are associated with skin extra-intestinal manifestations in CD. Its highest expression was detected in human skin, in psoriasis and in vitiligo. Polymorphisms of NLRP1 were found in lupus erythematosus and diabetes type 1. Variants of mouse NLRP1 were found to be activated upon N-terminal cleavage by the protease in anthrax lethal factor.
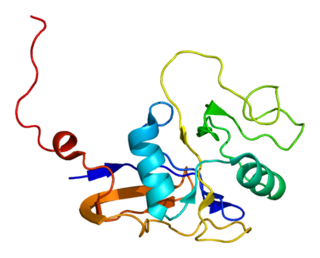
Anthrax toxin receptor 2 is a protein that in humans is encoded by the ANTXR2 gene.

Ilomastat (INN), is a broad-spectrum matrix metalloproteinase inhibitor.
Bradley Lether Pentelute is currently a professor of chemistry at the Massachusetts Institute of Technology (MIT). His research program lies at the intersection of chemistry and biology and develops bioconjugation strategies, cytosolic delivery platforms, and rapid flow synthesis technologies to optimize the production, achieve site-specific modification, enhance stability, and modulate function of a variety of bioactive agents. His laboratory successfully modified proteins via cysteine-containing “pi-clamps” made up of a short sequence of amino acids, and delivered large biomolecules, such as various proteins and drugs, into cells via the anthrax delivery vehicle. Pentelute has also made several key contributions to automated synthesis technologies in flow. These advances includes the invention of the world's fastest polypeptide synthesizer. This system is able to form amide bonds at a more efficient rate than standard commercial equipment and has helped in the process of understanding protein folding and its mechanisms. This automated flow technology was recently used to achieve total chemical synthesis of protein chains up to 164 amino acids in length that retained the structure and function of native variants obtained by recombinant expression. The primary goal of his endeavor is to use these processes to create designer biologics that can be used to treat diseases and solve the manufacturing problem for on-demand personalized therapies, such as cancer vaccines.