
An endosymbiont or endobiont is an organism that lives within the body or cells of another organism. Typically the two organisms are in a mutualistic relationship. Examples are nitrogen-fixing bacteria, which live in the root nodules of legumes, single-cell algae inside reef-building corals, and bacterial endosymbionts that provide essential nutrients to insects.

Rickettsia is a genus of nonmotile, gram-negative, nonspore-forming, highly pleomorphic bacteria that may occur in the forms of cocci, bacilli, or threads. The genus was named after Howard Taylor Ricketts in honor of his pioneering work on tick-borne spotted fever.

Wolbachia is a genus of gram-negative bacteria infecting many species of arthropods and filarial nematodes. The symbiotic relationship ranges from parasitism to obligate mutualism. It is one of the most common parasitic microbes of arthropods, and is possibly the most widespread reproductive parasite bacterium in the biosphere. Its interactions with hosts are complex and highly diverse across different host species. Some host species cannot reproduce, or even survive, without Wolbachia colonisation. One study concluded that more than 16% of neotropical insect species carry bacteria of this genus, and as many as 25 to 70% of all insect species are estimated to be potential hosts.
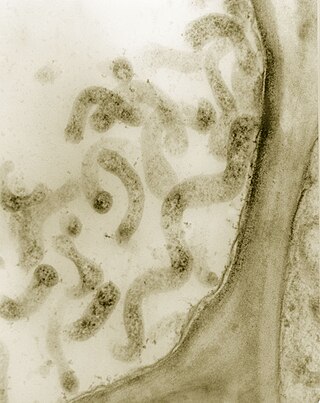
Spiroplasma is a genus of Mollicutes, a group of small bacteria without cell walls. Spiroplasma shares the simple metabolism, parasitic lifestyle, fried-egg colony morphology and small genome of other Mollicutes, but has a distinctive helical morphology, unlike Mycoplasma. It has a spiral shape and moves in a corkscrew motion. Many Spiroplasma are found either in the gut or haemolymph of insects where they can act to manipulate host reproduction, or defend the host as endosymbionts. Spiroplasma are also disease-causing agents in the phloem of plants. Spiroplasmas are fastidious organisms, which require a rich culture medium. Typically they grow well at 30 °C, but not at 37 °C. A few species, notably Spiroplasma mirum, grow well at 37 °C, and cause cataracts and neurological damage in suckling mice. The best studied species of spiroplasmas are Spiroplasma poulsonii, a reproductive manipulator and defensive insect symbiont, Spiroplasma citri, the causative agent of citrus stubborn disease, and Spiroplasma kunkelii, the causative agent of corn stunt disease.
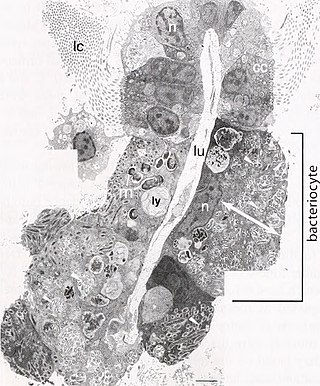
A bacteriocyte, also known as a mycetocyte, is a specialized adipocyte found primarily in certain insects such as aphids, tsetse flies, German cockroaches, weevils, and ants. These cells contain endosymbiotic organisms such as bacteria and fungi, which provide essential amino acids and other chemicals to their host. Bacteriocytes may aggregate into a specialized organ called the bacteriome.

Nasonia are a genus of small pteromalid parasitoid wasps that sting and lay eggs in the pupae of various flies. The fly species that Nasonia usually parasitize are primarily blow flies and flesh flies, making Nasonia a useful tool for biocontrol of these pest flies. The small match-head sized wasps are also referred to as jewel wasps based on the emerald sheen of their exoskeleton.

Photorhabdus luminescens is a Gammaproteobacterium of the family Morganellaceae, and is a lethal pathogen of insects.

Nasonia vitripennis is one of four known species under the genus Nasonia - small parasitoid wasps that afflict the larvae of parasitic carrion flies such as blowflies and flesh flies, which themselves are parasitic toward nestling birds. It is the best known and most widely studied of the parasitoid wasps, and their study forms a vital part of the information used to describe the order Hymenoptera, along with information from bees and ants. This parasitoid behaviour makes the wasps an interest for the development of biopesticide and biological systems for controlling unwanted insects.
Cytoplasmic incompatibility (CI) is a mating incompatibility reported in many arthropod species that is caused by intracellular parasites such as Wolbachia. These bacteria reside in the cytoplasm of the host cells and modify their hosts' sperm in a way that leads to embryo death unless this modification is 'rescued' by the same bacteria in the eggs. CI has been reported in many insect species, as well as in mites and woodlice. Aside from Wolbachia, CI can be induced by the bacteria Cardinium,Rickettsiella, Candidatus Mesenet longicola and Spiroplasma. CI is currently being exploited as a mechanism for Wolbachia-mediated disease control in mosquitoes.

Acyrthosiphon pisum, commonly known as the pea aphid, is a sap-sucking insect in the family Aphididae. It feeds on several species of legumes worldwide, including forage crops, such as pea, clover, alfalfa, and broad bean, and ranks among the aphid species of major agronomical importance. The pea aphid is a model organism for biological study whose genome has been sequenced and annotated.
Nasonia longicornis is a species of pteromalid wasp in the family Pteromalidae. It can be identified by the structure of its antennae. It is a parasitoid of Protocalliphora pupae, usually found in birds' nests. The species is found in western North America. Females usually only mate once in their lifetime.
Photorhabdus is a genus of bioluminescent, gram-negative bacilli which lives symbiotically within entomopathogenic nematodes, hence the name photo and rhabdus. Photorhabdus is known to be pathogenic to a wide range of insects and has been used as biopesticide in agriculture.
Hamiltonella defensa is a species of bacteria. It is maternally or sexually transmitted and lives as an endosymbiont of whiteflies and aphids, meaning that it lives within a host, protecting its host from attack. It does this through bypassing the host's immune responses by protecting its host against parasitoid wasps. However, H. defensa is only defensive if infected by a virus. H. defensa shows a relationship with Photorhabdus species, together with Regiella insecticola. Together with other endosymbionts, it provides aphids protection against parasitoids. It is known to habitate Bemisia tabaci.
Arsenophonus is a genus of Morganellaceae, of the Gammaproteobacteria. Members of the Arsenophonus genus are increasingly discovered bacterial symbionts of arthropods that are estimated to infect over 5% of arthropod species globally and form a variety of relationships with hosts across the mutualism parasitism continuum. Arsenophonus bacteria have been identified in a diversity of insect taxa, including economically important species such as the Western honey bee and the rice pest Nilaparvata lugens.
Spiroplasma poulsonii are bacteria of the genus Spiroplasma that are commonly endosymbionts of flies. These bacteria live in the hemolymph of the flies, where they can act as reproductive manipulators or defensive symbionts.

The Drosophila quinaria species group is a speciose lineage of mushroom-feeding flies studied for their specialist ecology, their parasites, population genetics, and the evolution of immune systems. Quinaria species are part of the Drosophila subgenus.
Vertical transmission of symbionts is the transfer of a microbial symbiont from the parent directly to the offspring. Many metazoan species carry symbiotic bacteria which play a mutualistic, commensal, or parasitic role. A symbiont is acquired by a host via horizontal, vertical, or mixed transmission.

The Morganellaceae are a family of Gram-negative bacteria that include some important human pathogens formerly classified as Enterobacteriaceae. This family is a member of the order Enterobacterales in the class Gammaproteobacteria of the phylum Pseudomonadota. Genera in this family include the type genus Morganella, along with Arsenophonus, Cosenzaea, Moellerella, Photorhabdus, Proteus, Providencia and Xenorhabdus.
Candidatus Arsenophonus arthropodicus is a Gram-negative and intracellular secondary (S) endosymbiont that belongs to the genus Arsenophonus. This bacterium is found in the Hippoboscid louse fly, Pseudolynchia canariensis. S-endosymbionts are commonly found in distinct tissues. Strains of recovered Arsenophonus found in arthropods share 99% sequence identification in the 16S rRNA gene across all species. Arsenophonus-host interactions involve parasitism and mutualism, including a popular mechanism of "male-killing" found commonly in a related species, Arsenophonus nasoniae. This species is considered "Ca. A. arthropodicus" due it being as of yet uncultured.

Alois M. Huger was a German entomologist and a pioneer in insect pathology as well as in the use of insect pathogens for the biological control of pest insects. He worked mainly on the diagnosis of insect diseases in Darmstadt, Germany. Among others, he discovered a virus disease of the coconut palm rhinoceros beetle in Malaysia from a previously unknown group of viruses which provided long-term control of this pest when introduced into islands invaded by the beetle.










