Ontogeny is the origination and development of an organism, usually from the time of fertilization of the egg to adult. The term can also be used to refer to the study of the entirety of an organism's lifespan.

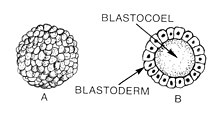
An embryo is the initial stage of development for a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.
Mammalian embryogenesis is the process of cell division and cellular differentiation during early prenatal development which leads to the development of a mammalian embryo.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity or lumen known as the blastocoel. In the late blastocyst, the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother. The corresponding structure in non-mammalian animals is an undifferentiated ball of cells called the blastula.

Drosophila embryogenesis, the process by which Drosophila embryos form, is a favorite model system for genetics and developmental biology. The study of its embryogenesis unlocked the century-long puzzle of how development was controlled, creating the field of evolutionary developmental biology. The small size, short generation time, and large brood size make it ideal for genetic studies. Transparent embryos facilitate developmental studies. Drosophila melanogaster was introduced into the field of genetic experiments by Thomas Hunt Morgan in 1909.
In biology, a blastomere is a type of cell produced by cell division (cleavage) of the zygote after fertilization; blastomeres are an essential part of blastula formation, and blastocyst formation in mammals.

The blastocoel, also spelled blastocoele and blastocele, and also called cleavage cavity, or segmentation cavity is a fluid-filled or yolk-filled cavity that forms in the blastula during very early embryonic development. At this stage in mammals the blastula is called the blastocyst, which consists of an outer epithelium, the trophectoderm, enveloping the inner cell mass and the blastocoel.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.
In embryology, cleavage is the division of cells in the early development of the embryo, following fertilization. The zygotes of many species undergo rapid cell cycles with no significant overall growth, producing a cluster of cells the same size as the original zygote. The different cells derived from cleavage are called blastomeres and form a compact mass called the morula. Cleavage ends with the formation of the blastula, or of the blastocyst in mammals.

The inner cell mass (ICM) or embryoblast is a structure in the early development of an embryo. It is the mass of cells inside the blastocyst that will eventually give rise to the definitive structures of the fetus. The inner cell mass forms in the earliest stages of embryonic development, before implantation into the endometrium of the uterus. The ICM is entirely surrounded by the single layer of trophoblast cells of the trophectoderm.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.
In developmental biology, midblastula or midblastula transition (MBT) occurs during the blastula stage of embryonic development in non-mammals. During this stage, the embryo is referred to as a blastula. The series of changes to the blastula that characterize the midblastula transition include activation of zygotic gene transcription, slowing of the cell cycle, increased asynchrony in cell division, and an increase in cell motility.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Human embryonic development covers the first eight weeks of development, which have 23 stages, called Carnegie stages. At the beginning of the ninth week, the embryo is termed a fetus. In comparison to the embryo, the fetus has more recognizable external features and a more complete set of developing organs.

Cavitation is a process in early embryonic development that follows cleavage. Cavitation is the formation of the blastocoel, a fluid-filled cavity that defines the blastula, or in mammals the blastocyst. After fertilization, cell division of the zygote occurs which results in the formation of a solid ball of cells (blastomeres) called the morula. Further division of cells increases their number in the morula, and the morula differentiates them into two groups. The internal cells become the inner cell mass, and the outer cells become the trophoblast. Before cell differentiation takes place there are two transcription factors, Oct-4 and nanog that are uniformly expressed on all of the cells, but both of these transcription factors are turned off in the trophoblast once it has formed.
Maternal to zygotic transition (MZT), also known as embryonic genome activation, is the stage in embryonic development during which development comes under the exclusive control of the zygotic genome rather than the maternal (egg) genome. The egg contains stored maternal genetic material mRNA which controls embryo development until the onset of MZT. After MZT the diploid embryo takes over genetic control. This requires both zygotic genome activation (ZGA), and degradation of maternal products. This process is important because it is the first time that the new embryonic genome is utilized and the paternal and maternal genomes are used in combination. The zygotic genome now drives embryo development.
Sir Richard Lavenham Gardner, FRSB, FRS is a British embryologist and geneticist. He is currently an Emeritus Professor at the University of York, and was previously a Royal Society Research Professor.
Magdalena Żernicka-Goetz is a Polish-British developmental biologist. She is Professor of Mammalian Development and Stem Cell Biology in the Department of Physiology, Development and Neuroscience and Fellow of Sidney Sussex College, Cambridge. She also serves as Bren Professor of Biology and Biological Engineering at California Institute of Technology (Caltech).

Smaug is a RNA-binding protein in Drosophila that helps in maternal to zygotic transition (MZT). The protein is named after the fictional character Smaug, the dragon in J.R.R. Tolkien's 1937 novel The Hobbit. The MZT ends with the midblastula transition (MBT), which is defined as the first developmental event in Drosophila that depends on zygotic mRNA. In Drosophila, the initial developmental events are controlled by maternal mRNAs like Hsp83, nanos, string, Pgc, and cyclin B mRNA. Degradation of these mRNAs, which is expected to terminate maternal control and enable zygotic control of embryogenesis, happens at interphase of nuclear division cycle 14. During this transition smaug protein targets the maternal mRNA for destruction using miRs. Thus activating the zygotic genes. Smaug is expected to play a role in expression of three miRNAs – miR-3, miR-6, miR-309 and miR-286 during MZT in Drosophila. Among them smaug dependent expression of miR-309 is needed for destabilization of 410 maternal mRNAs. In smaug mutants almost 85% of maternal mRNA is found to be stable. Smaug also binds to 3′ untranslated region (UTR) elements known as SMG response elements (SREs) on nanos mRNA and represses its expression by recruiting a protein called Cup(an eIF4E-binding protein that blocks the binding of eIF4G to eIF4E). There after it recruits deadenylation complex CCR4-Not on to the nanos mRNA which leads to deadenylation and subsequent decay of the mRNA. It is also found to be involved in degradation and repression of maternal Hsp83 mRNA by recruiting CCR4/POP2/NOT deadenylase to the mRNA. The human Smaug protein homologs are SAMD4A and SAMD4B.
This glossary of developmental biology is a list of definitions of terms and concepts commonly used in the study of developmental biology and related disciplines in biology, including embryology and reproductive biology, primarily as they pertain to vertebrate animals and particularly to humans and other mammals. The developmental biology of invertebrates, plants, fungi, and other organisms is treated in other articles; e.g terms relating to the reproduction and development of insects are listed in Glossary of entomology, and those relating to plants are listed in Glossary of botany.