
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.

The genitourinary system, or urogenital system, are the organs of the reproductive system and the urinary system. These are grouped together because of their proximity to each other, their common embryological origin and the use of common pathways, like the male urethra. Also, because of their proximity, the systems are sometimes imaged together.

Somitogenesis is the process by which somites form. Somites are bilaterally paired blocks of paraxial mesoderm that form along the anterior-posterior axis of the developing embryo in segmented animals. In vertebrates, somites give rise to skeletal muscle, cartilage, tendons, endothelium, and dermis.
The development of the urinary system begins during prenatal development, and relates to the development of the urogenital system – both the organs of the urinary system and the sex organs of the reproductive system. The development continues as a part of sexual differentiation.

The mesonephros is one of three excretory organs that develop in vertebrates. It serves as the main excretory organ of aquatic vertebrates and as a temporary kidney in reptiles, birds, and mammals. The mesonephros is included in the Wolffian body after Caspar Friedrich Wolff who described it in 1759.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians, the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation, the primitive streak extends for half the length of the embryo. In the human embryo, this appears by stage 6, about 17 days.
Kidney development, or nephrogenesis, describes the embryologic origins of the kidney, a major organ in the urinary system. This article covers a 3 part developmental process that is observed in most reptiles, birds and mammals, including humans. Nephrogenesis is often considered in the broader context of the development of the urinary and reproductive organs.
Pronephros is the most basic of the three excretory organs that develop in vertebrates, corresponding to the first stage of kidney development. It is succeeded by the mesonephros, which in fish and amphibians remains as the adult kidney. In amniotes, the mesonephros is the embryonic kidney and a more complex metanephros acts as the adult kidney. Once a more advanced kidney forms, the previous version typically degenerates by apoptosis or becomes part of the male reproductive system.
The ureteric bud, also known as the metanephric diverticulum, is a protrusion from the mesonephric duct during the development of the urinary and reproductive organs. It later develops into a conduit for urine drainage from the kidneys, which, in contrast, originate from the metanephric blastema.
The metanephrogenic blastema or metanephric blastema is one of the two embryological structures that give rise to the kidney, the other being the ureteric bud.
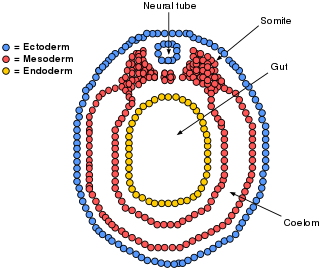
The lateral plate mesoderm is the mesoderm that is found at the periphery of the embryo. It is to the side of the paraxial mesoderm, and further to the axial mesoderm. The lateral plate mesoderm is separated from the paraxial mesoderm by a narrow region of intermediate mesoderm. The mesoderm is the middle layer of the three germ layers, between the outer ectoderm and inner endoderm.

Paraxial mesoderm, also known as presomitic or somitic mesoderm, is the area of mesoderm in the neurulating embryo that flanks and forms simultaneously with the neural tube. The cells of this region give rise to somites, blocks of tissue running along both sides of the neural tube, which form muscle and the tissues of the back, including connective tissue and the dermis.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.
The limb bud is a structure formed early in vertebrate limb development. As a result of interactions between the ectoderm and underlying mesoderm, formation occurs roughly around the fourth week of development. In the development of the human embryo the upper limb bud appears in the third week and the lower limb bud appears four days later.
Gremlin is an inhibitor in the TGF beta signaling pathway. It primarily inhibits bone morphogenesis and is implicated in disorders of increased bone formation and several cancers.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks have 23 stages.
The development of the reproductive system is the part of embryonic growth that results in the sex organs and contributes to sexual differentiation. Due to its large overlap with development of the urinary system, the two systems are typically described together as the genitourinary system.

Protein odd-skipped-related 1 is a transcription factor that in humans is encoded by the OSR1 gene. The OSR1 and OSR2 transcription factors participate in the normal development of body parts such as the kidney.
A mesenchymal–epithelial transition (MET) is a reversible biological process that involves the transition from motile, multipolar or spindle-shaped mesenchymal cells to planar arrays of polarized cells called epithelia. MET is the reverse process of epithelial–mesenchymal transition (EMT) and it has been shown to occur in normal development, induced pluripotent stem cell reprogramming, cancer metastasis and wound healing.
The kidneys are a pair of organs of the excretory system in vertebrates, which maintain the balance of water and electrolytes in the body (osmoregulation), filter the blood, remove metabolic waste products, and, in many vertebrates, also produce hormones and maintain blood pressure. In healthy vertebrates, the kidneys maintain homeostasis of extracellular fluid in the body. When the blood is being filtered, the kidneys form urine, which consists of water and excess or unnecessary substances, the urine is then excreted from the body through other organs, which in vertebrates, depending on the species, may include the ureter, urinary bladder, cloaca, and urethra.










