Bone morphogenetic protein 15 (BMP-15) is a protein that in humans is encoded by the BMP15 gene. It is involved in folliculogenesis, the process in which primordial follicles develop into pre-ovulatory follicles.
Bone morphogenetic protein 15 (BMP-15) is a protein that in humans is encoded by the BMP15 gene. It is involved in folliculogenesis, the process in which primordial follicles develop into pre-ovulatory follicles.
The BMP-15 gene is located on the X-chromosome and using Northern blot analysis BMP-15 mRNA is locally expressed within the ovaries in oocytes only after they have started to undergo the primary stages of development. [5] [6] BMP-15 is translated as a preproprotein that is composed of a single peptide, which contains a proregion and a smaller mature region. [6] Intracellular processing then leads to the removal of the proregion, leaving the biologically active mature region to perform the functions. [5] This protein is a member of the Transforming growth factor beta (TGF-β) superfamily and is a paracrine signalling molecule. [7] Most active BMPs have a common structure, in which they contain 7 cysteines, 6 of which form three intramolecular disulphide bonds and the seventh being involved in the formation of dimers with other monomers. [7] BMP-15 is an exception to this as the molecule does not contain the seventh cysteine. [7] Instead in BMP-15 the fourth cysteine is replaced by a serine. [7]
BMP-15 and GDF9 interact with each other and work synergistically to have similar interactions with the target cell. [8] BMP15 can act as a heterodimer with GDF9 or on its own as a homodimer. [8] In most of the BMP family heterodimers and homodimers form as the seventh cysteine is involved in the formation of a covalent bond, leading the dimerization. [9] However, in the BMP-15 the homodimers form as a non-covalent bond is present between two BMP-15 subunits. [7]
Functions of BMP-15 include [10]
Folliculogenesis is an important process for the development and maintenance of fertility. Primordial follicles are stored in the ovary and throughout life are activated to go through morphological changes to become preovulatory follicles ready for ovulation, when the oocyte is released into the fallopian tube of the female reproductive tract. [11]
BMP-15 main functions are crucial for the beginning of folliculogenesis as seen in Image 1. The primordial follicle is made up of the oocyte and a single layer of flattened granulosa cells. BMP-15 is released from the oocyte into the surrounding granulosa tissue where it binds to two membrane bound receptors on granulosa cells. [9] This promotes granulosa cell proliferation via mitosis. BMP-15 promotes the change of primordial to primary and secondary follicles which are surrounded by several granulosa cell layers but doesn't promote transition into preovulatory follicles. [12]

BMP-15 prevents differentiation into preovulatory follicle by inhibiting FSH action in granulosa. FSH is released by the anterior pituitary as part of the hypothalamic-pituitary-gonadal axis and promotes the differentiation of early follicles into later preovulatory ones. BMP-15 prevents this transition by inhibiting the production of FSH receptor mRNA in granulosa cells. Therefore, FSH cannot bind to the granulosa cells, this inhibits FSH dependent progesterone production and luteinization, subsequently granulosa cells do not differentiate. [12] [8]
As BMP-15 acts directly on granulosa cells it has an important influence on granulosa function including steroidogenesis inhibition of luteinization and differentiation of cumulus, without which would lead to infertility and lack of folliculogenesis. [13]
The use of mammalian species other than human is often used in research to learn more about human biology.
Two breeds of sheep, Inverdale and Hanna, are naturally heterozygous carriers of point mutations in the BMP-15 gene. [9] These point mutations result in higher ovulation rates and larger litter sizes than sheep strains with a wildtype BMP-15 genotype. [9] This super-fertility was mimicked later through immunization of wildtype ewes against BMP-15 using various immunisation techniques. [9] Sheep carrying homozygous alleles for the Inverdale and Hanna BMP-15 mutations are infertile, as they have streak ovaries and the primary stage of folliculogenesis is inhibited. [9] These studies suggest that BMP-15 plays a vital role in the normal regulation of folliculogenesis and ovulation in sheep. [12]
In mice, the BMP-15 homologue is not as physiologically important. [9] Upon targeted deletion of a bmp15 exon, the mice presented with only subfertility in homozygotes and no clear aberrant phenotype in heterozygotes. [9] The homozygous mutant mice did not suffer from reduced folliculogenesis or impacted follicle progression, unlike in the sheep homologue knockout experiments. [9] The subfertility seen in the homozygous mutant phenotype was attributed to defective ovulation and reduced viability of embryos. Here it can be stated that BMP-15 is not as vital for normal female mouse fertility as it is for sheep. [9]
Humans display a similar phenotype to the Inverdale/Hanna sheep in regards to female fertility. [9] In women, a mutation in BMP-15 is linked to hypergonadotropic ovarian failure due to ovarian dysgenesis. [9] In this case, the researchers were able to identify that the father of the two sisters displaying this mutation had no documented phenotype associated with the mutation, so BMP-15 appears to only affect females. [9] In slight contrast to the reports on sheep, the women in this study were heterozygous for the BMP-15 mutation but exhibited streak ovaries, a phenotype very similar to the one seen in homozygous mutant ewes. [9] The sisters presented with primary amenorrhea, showing that BMP-15 is also vital to normal human female fertility, concordant with the sheep model. [9]
The main theory for this stark difference between mammalian species relates to the number of follicles normally ovulated in each cycle by each species. [9] Humans and sheep are mono-ovulatory, potentially explaining the difference in litter size observed in mutant individuals. [9] As mice are poly-ovulatory, the role of BMP-15 in female mouse fertility may not be as obvious. [9]
Mutations within the gene for BMP-15 have been associated with reproductive complications in females, due to the X-linked nature of the protein. Due to its role in folliculogenesis, mutations can lead to sub-fertility through decreased or absent folliculogenesis. In combination with GDF-9, mutant BMP-15 is also associated with ovulation defects, premature ovarian failure and other reproductive pathologies. [13]
BMP-15 defects have been implicated in female sterility, Polycystic Ovary Syndrome (PCOS), primary ovarian insufficiency (POI) and endometriosis. Women with PCOS have been noted to have higher levels of BMP-15, [8] while missense mutations of the protein have been identified in females with POI. [9]
Research has also found inherited mutant BMP-15 to be involved with the pathogenesis of hypergonadotropic ovarian failure. [8] This condition develops due to BMP-15 role in folliculogenesis, and the errors that occur when a mutant gene is inherited. The protein is linked to familial ovarian dysgenesis which results in hypergonadotropic ovarian failure. [8]
The importance of BMP-15 in ovulation and folliculogenesis has been highlighted by research into Turner syndrome, a chromosomal abnormality where females are missing a complete or partial X chromosome. Depending on the chromosomal mutation, BMP-15 gene dosage varies and impacts ovarian development in Turner syndrome patients. The gene is thus involved in determining the extent of the ovarian defects present in Turner syndrome. [9]
BMP-15 is also present in animals and involved in reproduction, such as in mice and sheep. Reduced levels of BMP-15 in sheep have shown to increase ovulation, leading to larger litter sizes. [9]

The ovary is an organ in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus. There is an ovary found on the left and the right side of the body. The ovaries also secrete hormones that play a role in the menstrual cycle and fertility. The ovary progresses through many stages beginning in the prenatal period through menopause. It is also an endocrine gland because of the various hormones that it secretes.

Ovulation is the release of eggs from the ovaries. In women, this event occurs when the ovarian follicles rupture and release the secondary oocyte ovarian cells. After ovulation, during the luteal phase, the egg will be available to be fertilized by sperm. In addition, the uterine lining (endometrium) is thickened to be able to receive a fertilized egg. If no conception occurs, the uterine lining as well as the egg will be shed during menstruation.

A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.

Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.

An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. At the time of puberty, women have approximately 200,000 to 300,000 follicles, each with the potential to release an egg cell (ovum) at ovulation for fertilization. These eggs are developed once every menstrual cycle with around 450–500 being ovulated during a woman's reproductive lifetime.

A granulosa cell or follicular cell is a somatic cell of the sex cord that is closely associated with the developing female gamete in the ovary of mammals.

In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte. Folliculogenesis describes the progression of a number of small primordial follicles into large preovulatory follicles that occurs in part during the menstrual cycle.

The hypothalamic–pituitary–gonadal axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. Because these glands often act in concert, physiologists and endocrinologists find it convenient and descriptive to speak of them as a single system.

Ovarian reserve is a term that is used to determine the capacity of the ovary to provide egg cells that are capable of fertilization resulting in a healthy and successful pregnancy. With advanced maternal age the number of egg cell that can be successfully recruited for a possible pregnancy declines, constituting a major factor in the inverse correlation between age and female fertility.

Growth/differentiation factor 9 is a protein that in humans is encoded by the GDF9 gene.

The follicular phase, also known as the preovulatory phase or proliferative phase, is the phase of the estrous cycle during which follicles in the ovary mature from primary follicle to a fully mature graafian follicle. It ends with ovulation. The main hormones controlling this stage are secretion of gonadotropin-releasing hormones, which are follicle-stimulating hormones and luteinising hormones. They are released by pulsatile secretion. The duration of the follicular phase can differ depending on the length of the menstrual cycle, while the luteal phase is usually stable, does not really change and lasts 14 days.
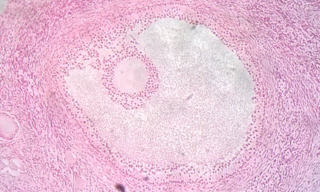
Follicular atresia refers to the process in which a follicle fails to develop, thus preventing it from ovulating and releasing an egg. It is a normal, naturally occurring progression that occurs as mammalian ovaries age. Approximately 1% of mammalian follicles in ovaries undergo ovulation and the remaining 99% of follicles go through follicular atresia as they cycle through the growth phases. In summary, follicular atresia is a process that leads to the follicular loss and loss of oocytes, and any disturbance or loss of functionality of this process can lead to many other conditions.

Bone morphogenetic protein receptor type II or BMPR2 is a serine/threonine receptor kinase encoded by the BMPR2 gene. It binds bone morphogenetic proteins, members of the TGF beta superfamily of ligands, which are involved in paracrine signaling. BMPs are involved in a host of cellular functions including osteogenesis, cell growth and cell differentiation. Signaling in the BMP pathway begins with the binding of a BMP to the type II receptor. This causes the recruitment of a BMP type I receptor, which the type II receptor phosphorylates. The type I receptor phosphorylates an R-SMAD, a transcriptional regulator.
The theca folliculi comprise a layer of the ovarian follicles. They appear as the follicles become secondary follicles.

An antral follicle, also known as Graafian follicle and tertiary follicle, is an ovarian follicle during a certain latter stage of folliculogenesis.

In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.

Homeobox protein NOBOX, also known as newborn ovary homeobox protein, is a protein that in humans is encoded by the NOBOX gene. The official symbol (NOBOX) and the official full name are maintained by the HGNC. The NOBOX gene is conserved in chimpanzee, Rhesus monkey, cow, mouse, and rat. There are 175 organisms that have orthologs with human gene NOBOX. It is capable of regulating other genes that are important in the development of follicles. Follicles do not develop and oocytes decrease in its absence which lead to infertility.
Follicle-stimulating hormone (FSH) insensitivity, or ovarian insensitivity to FSH in females, also referable to as ovarian follicle hypoplasia or granulosa cell hypoplasia in females, is a rare autosomal recessive genetic and endocrine syndrome affecting both females and males, with the former presenting with much greater severity of symptomatology. It is characterized by a resistance or complete insensitivity to the effects of follicle-stimulating hormone (FSH), a gonadotropin which is normally responsible for the stimulation of estrogen production by the ovaries in females and maintenance of fertility in both sexes. The condition manifests itself as hypergonadotropic hypogonadism, reduced or absent puberty, amenorrhea, and infertility in females, whereas males present merely with varying degrees of infertility and associated symptoms.
Ovarian follicle activation can be defined as primordial follicles in the ovary moving from a quiescent (inactive) to a growing phase. The primordial follicle in the ovary is what makes up the “pool” of follicles that will be induced to enter growth and developmental changes that change them into pre-ovulatory follicles, ready to be released during ovulation. The process of development from a primordial follicle to a pre-ovulatory follicle is called folliculogenesis.
Ovarian follicle dominance is the process where one or more follicles are selected per cycle to ovulate.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.