In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

Carl Dietrich Harries was a German chemist born in Luckenwalde, Brandenburg, Prussia. He received his doctorate in 1892. In 1900, he married Hertha von Siemens, daughter of the electrical genius Werner von Siemens, and the inventor of one of the earliest ozone generators. In 1904, he moved as full professor to the University of Kiel, where he remained until 1916. During that time he published numerous papers on ozonolysis. His major publication detailing ozonolysis was published in Liebigs Ann. Chem. 1905, 343, 311. Dissatisfied with academic life and having failed to obtain either of two positions at universities, he left academia to become Director of Research at Siemens and Halske. He died on 3 November 1923 of complications following surgery for cancer.

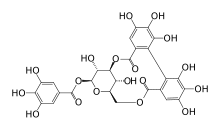
Punicalagin is an ellagitannin, a type of phenolic compound. It is found in forms alpha and beta in pomegranates, in Terminalia catappa and Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow, a plant species found in South Africa. These three genera are all Myrtales and the last two are both Combretaceae.
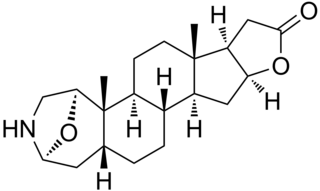
Samandaridine is an extremely toxic alkaloid produced by the skin glands of various salamanders.
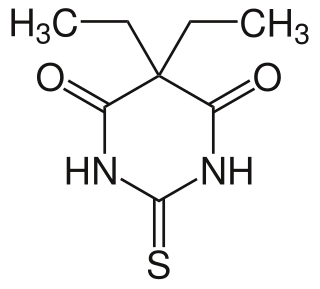
Thiobarbital is a drug which is a barbiturate derivative. It is the thiobarbiturate analogue of barbital.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed.The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.

Carbonic anhydrase inhibitors are a class of pharmaceuticals that suppress the activity of carbonic anhydrase. Their clinical use has been established as anti-glaucoma agents, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, idiopathic intracranial hypertension, neurological disorders, or osteoporosis.
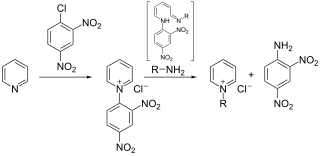
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke.

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:
Walter Julius Reppe was a German chemist. He is notable for his contributions to the chemistry of acetylene.

Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser.

The Rosenmund–von Braun synthesis is an organic reaction in which an aryl halide reacts with cuprous cyanide to yield an aryl nitrile.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.

Heinrich Debus was a German chemist.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.

Granatin A is an ellagitannin found in the pericarp of Punica granatum (pomegranate). It is a weak carbonic anhydrase inhibitor.

Granatin B is an ellagitannin found in the fruit of Punica granatum (pomegranate). It is a molecule having an enantiomeric dehydrohexahydroxydiphenoyl group.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Casuarinin is an ellagitannin. It is found in the pericarp of pomegranates. It is also found in Casuarina and Stachyurus species and in Alnus sieboldiana.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.