
A scar is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs and tissues of the body. Thus, scarring is a natural part of the healing process. With the exception of very minor lesions, every wound results in some degree of scarring. An exception to this are animals with complete regeneration, which regrow tissue without scar formation.

The prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from the fatty acid arachidonic acid. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
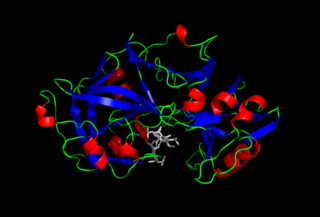
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the stomach and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site.

In biology, the extracellular matrix (ECM) is a three-dimensional network of extracellular macromolecules, such as collagen, enzymes, and glycoproteins, that provide structural and biochemical support of surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
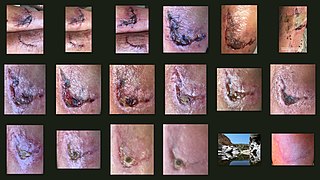
Wound healing is a complex process in which the skin, and the tissues under it, repair themselves after injury. In this article, wound healing is depicted in a discrete timeline of physical attributes (phases) constituting the post-trauma repairing process. In undamaged skin, the epidermis and dermis form a protective barrier against the external environment. When the barrier is broken, a regulated sequence of biochemical events is set into motion to repair the damage. This process is divided into predictable phases: blood clotting (hemostasis), inflammation, tissue growth (proliferation), and tissue remodeling (maturation). Blood clotting may be considered to be part of the inflammation stage instead of a separate stage.
Protease inhibitors (PIs) are a class of antiviral drugs that are widely used to treat HIV/AIDS and hepatitis C. Protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Concanavalin A (ConA) is a lectin originally extracted from the jack-bean, Canavalia ensiformis. It is a member of the legume lectin family. It binds specifically to certain structures found in various sugars, glycoproteins, and glycolipids, mainly internal and nonreducing terminal α-D-mannosyl and α-D-glucosyl groups. ConA is a plant mitogen, and is known for its ability to stimulate mouse T-cell subsets giving rise to four functionally distinct T cell populations, including precursors to suppressor T-cell; one subset of human suppressor T-cells as well is sensitive to ConA. ConA was the first lectin to be available on a commercial basis, and is widely used in biology and biochemistry to characterize glycoproteins and other sugar-containing entities on the surface of various cells. It is also used to purify glycosylated macromolecules in lectin affinity chromatography, as well as to study immune regulation by various immune cells.

Melphalan is a chemotherapy drug belonging to the class of nitrogen mustard alkylating agents.

A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAM-TS2) also known as procollagen I N-proteinase is an enzyme that in humans is encoded by the ADAMTS2 gene.

Bone morphogenetic protein 1, also known as BMP1, is a protein which in humans is encoded by the BMP1 gene. There are seven isoforms of the protein created by alternate splicing.

Maspin is a protein that in humans is encoded by the SERPINB5 gene. This protein belongs to the serpin superfamily. SERPINB5 was originally reported to function as a tumor suppressor gene in epithelial cells, suppressing the ability of cancer cells to invade and metastasize to other tissues. Furthermore, and consistent with an important biological function, Maspin knockout mice were reported to be non-viable, dying in early embryogenesis. However, a subsequent study using viral transduction as a method of gene transfer was not able to reproduce the original findings and found no role for maspin in tumour biology. Furthermore, the latter study demonstrated that maspin knockout mice are viable and display no obvious phenotype. These data are consistent with the observation that maspin is not expressed in early embryogenesis. The precise molecular function of maspin is thus currently unknown.

Collagen alpha-6(IV) chain is a protein that in humans is encoded by the COL4A6 gene.

Glia-derived nexin is a protein that in humans is encoded by the SERPINE2 gene.

Collagen alpha-1(XII) chain is a protein that in humans is encoded by the COL12A1 gene.
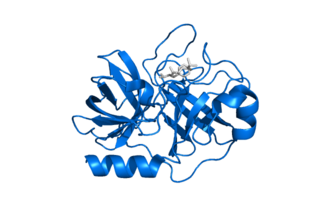
Kallikrein-related peptidase 7 (KLK7) is a serine protease that in humans is encoded by the KLK7 gene. KLK7 was initially purified from the epidermis and characterised as stratum corneum chymotryptic enzyme (SCCE). It was later identified as the seventh member of the human kallikrein family, which includes fifteen homologous serine proteases located on chromosome 19 (19q13).

Collagen alpha-1(XXV) chain is a protein that in humans is encoded by the COL25A1 gene.
Keratinases are proteolytic enzymes that digest keratin.
Gene transfer strategies for the potential medical management of osteoarthritis are under preliminary research to define pathological mechanisms and possible treatments for this chronic disease. Unlike pharmacological treatments which are administered systemically, gene therapy aims to establish sustained, synthesis of gene products and tissue rehabilitation within the joint.

















