
Adenylate cyclase is an enzyme with systematic name ATP diphosphate-lyase . It catalyzes the following reaction:
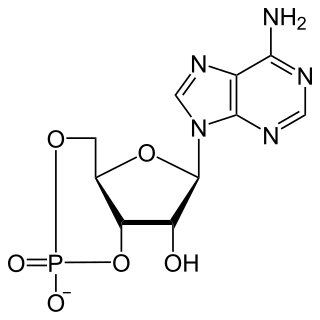
Cyclic adenosine monophosphate is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.

Riboflavin, also known as vitamin B2, is a vitamin found in food and sold as a dietary supplement. It is essential to the formation of two major coenzymes, flavin mononucleotide and flavin adenine dinucleotide. These coenzymes are involved in energy metabolism, cellular respiration, and antibody production, as well as normal growth and development. The coenzymes are also required for the metabolism of niacin, vitamin B6, and folate. Riboflavin is prescribed to treat corneal thinning, and taken orally, may reduce the incidence of migraine headaches in adults.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.

In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.

Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as cofactor in biological blue-light photo receptors. During the catalytic cycle, a reversible interconversion of the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms occurs in the various oxidoreductases. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization.

Cyclic ADP Ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide (like cAMP) with two phosphate groups present on 5' OH of the adenosine (like ADP), further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 (N1) of the same adenine base (whose position N9 has the glycosidic bond to the other ribose). The N1-glycosidic bond to adenine is what distinguishes cADPR from ADP-ribose (ADPR), the non-cyclic analog. cADPR is produced from nicotinamide adenine dinucleotide (NAD+) by ADP-ribosyl cyclases (EC 3.2.2.5) as part of a second messenger system.
In enzymology, an inositol-3-phosphate synthase is an enzyme that catalyzes the chemical reaction
The enzyme cytidylate cyclase is an enzyme that catalyzes the reaction
The enzyme glycosylphosphatidylinositol diacylglycerol-lyase catalyzes the reaction
The enzyme phosphatidylinositol diacylglycerol-lyase catalyzes the following reaction:
In enzymology, a RNA-3′-phosphate cyclase is an enzyme that catalyzes the chemical reaction
The enzyme taxadiene synthase catalyzes the chemical reaction
In enzymology, a FAD diphosphatase (EC 3.6.1.18) is an enzyme that catalyzes the chemical reaction
In enzymology, a [acetyl-CoA carboxylase] kinase is an enzyme that catalyzes the chemical reaction

In enzymology, a riboflavin kinase is an enzyme that catalyzes the chemical reaction

Triokinase/FMN cyclase is an enzyme that in humans is encoded by the DAK gene.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
Mn2+-dependent ADP-ribose/CDP-alcohol diphosphatase (EC 3.6.1.53, Mn2+-dependent ADP-ribose/CDP-alcohol pyrophosphatase, ADPRibase-Mn) is an enzyme with systematic name CDP-choline phosphohydrolase. This enzyme catalyses the following chemical reaction












