Related Research Articles

The eastern copperhead, also known simply as the copperhead, is a species of venomous snake, a pit viper, endemic to eastern North America; it is a member of the subfamily Crotalinae in the family Viperidae.

Agkistrodon is a genus of venomous pit vipers commonly known as American moccasins. The genus is endemic to North America, ranging from the Southern United States to northern Costa Rica. Eight species are currently recognized, all of them monotypic and closely related. Common names include: cottonmouths, copperheads, and cantils.
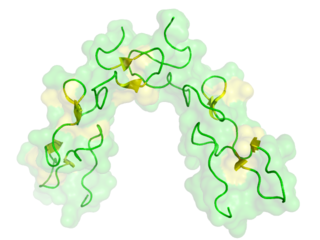
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

Agkistrodon laticinctus, commonly known as the broad-banded copperhead, is a venomous pit viper species, formerly considered a subspecies of Agkistrodon contortrix, which is found in the southern United States, from Kansas, through Oklahoma and throughout central Texas.
Adamalysin is an enzyme. This enzyme catalyses the following chemical reaction
Helge Stormorken was a Norwegian veterinarian and physician.

Cysteine-rich secretory proteins, often abbreviated as CRISPs, are a group of glycoproteins. They are a subgroup of the CRISP, antigen 5 and Pr-1 (CAP) protein superfamily and also contain a domain related to the ShK toxins. They are substantially implicated in the functioning of the mammalian reproductive system. CRISPs are also found in a variety of snake venoms where they inhibit both smooth muscle contraction and cyclic nucleotide-gated ion channels.
Venombin A is an enzyme. This enzyme catalyses the following chemical reaction
Snake venom factor V activator is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
Bothropasin is an enzyme. This enzyme catalyses the following chemical reaction
Leucolysin is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin C is an enzyme. This enzyme catalyses the following chemical reaction
Atroxase is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin E is an enzyme. This enzyme catalyses the following chemical reaction
Ruberlysin is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin I is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin II is an enzyme. This enzyme catalyses the following chemical reaction
Mucrolysin is an enzyme. This enzyme catalyses the following chemical reaction
Jararhagin is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Retzios AD, Markland FS (December 1988). "A direct-acting fibrinolytic enzyme from the venom of Agkistrodon contortrix contortrix: effects on various components of the human blood coagulation and fibrinolysis systems". Thrombosis Research. 52 (6): 541–52. doi:10.1016/0049-3848(88)90127-2. PMID 3232124.
- ↑ Guan AL, Retzios AD, Henderson GN, Markland FS (September 1991). "Purification and characterization of a fibrinolytic enzyme from venom of the southern copperhead snake (Agkistrodon contortrix contortrix)". Archives of Biochemistry and Biophysics. 289 (2): 197–207. doi:10.1016/0003-9861(91)90462-r. PMID 1898066.
- ↑ Randolph A, Chamberlain SH, Chu HL, Retzios AD, Markland FS, Masiarz FR (May 1992). "Amino acid sequence of fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon contortrix contortrix venom". Protein Science. 1 (5): 590–600. doi:10.1002/pro.5560010505. PMC 2142229 . PMID 1304358.
- ↑ Loayza SL, Trikha M, Markland FS, Riquelme P, Kuo J (December 1994). "Resolution of isoforms of natural and recombinant fibrolase, the fibrinolytic enzyme from Agkistrodon contortrix contortrix snake venom, and comparison of their EDTA sensitivities". Journal of Chromatography B. 662 (2): 227–43. doi:10.1016/0378-4347(94)00202-9. PMID 7719479.
- ↑ Retzios AD, Markland FS (May 1994). "Fibrinolytic enzymes from the venoms of Agkistrodon contortrix contortrix and Crotalus basiliscus basiliscus: cleavage site specificity towards the alpha-chain of fibrin". Thrombosis Research. 74 (4): 355–67. doi:10.1016/0049-3848(94)90151-1. PMID 8085237.