Related Research Articles

A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
Asparagine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain carboxamide, classifying it as a polar, aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.

Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.
A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.

Membrane alanyl aminopeptidase also known as alanyl aminopeptidase (AAP) or aminopeptidase N (AP-N) is an enzyme that in humans is encoded by the ANPEP gene.
Collagenases are enzymes that break the peptide bonds in collagen. They assist in destroying extracellular structures in the pathogenesis of bacteria such as Clostridium. They are considered a virulence factor, facilitating the spread of gas gangrene. They normally target the connective tissue in muscle cells and other body organs.

Asparagine synthetase is a chiefly cytoplasmic enzyme that generates asparagine from aspartate. This amidation reaction is similar to that promoted by glutamine synthetase. The enzyme is ubiquitous in its distribution in mammalian organs, but basal expression is relatively low in tissues other than the exocrine pancreas.

Nuclease S1 is an endonuclease enzyme that splits single-stranded DNA (ssDNA) and RNA into oligo- or mononucleotides. This enzyme catalyses the following chemical reaction

In enzymology, a peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase (EC 3.5.1.52) is an enzyme that catalyzes a chemical reaction that cleaves a N4-(acetyl-beta-D-glucosaminyl)asparagine residue in which the glucosamine residue may be further glycosylated, to yield a (substituted) N-acetyl-beta-D-glucosaminylamine and a peptide containing an aspartate residue. This enzyme belongs to the family of hydrolases, specifically those acting on carbon-nitrogen bonds other than peptide bonds in linear amides.

PNGase also known as N-glycanase 1 or peptide-N(4)-(N-acetyl-beta-glucosaminyl)asparagine amidase is an enzyme that in humans is encoded by the NGLY1 gene. PNGase is a de-N-glycosylating enzyme that removes N-linked or asparagine-linked glycans (N-glycans) from glycoproteins. More specifically, NGLY1 catalyzes the hydrolysis of the amide bond between the innermost N-acetylglucosamine (GlcNAc) and an Asn residue on an N-glycoprotein, generating a de-N-glycosylated protein, in which the N-glycoylated Asn residue is converted to asp, and a 1-amino-GlcNAc-containing free oligosaccharide. Ammonia is then spontaneously released from the 1-amino GlcNAc at physiological pH (<8), giving rise to a free oligosaccharide with an N,N’-diacetylchitobiose structure at the reducing end.
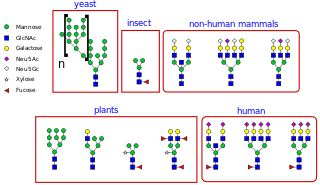
N-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.

Ecadotril is a neutral endopeptidase inhibitor ((NEP) EC 3.4.24.11) and determined by the presence of peptidase family M13 as a neutral endopeptidase inhibited by phosphoramidon. Ecadotril is the (S)-enantiomer of racecadotril. NEP-like enzymes include the endothelin-converting enzymes. The peptidase M13 family believed to activate or inactivate oligopeptide (pro)-hormones such as opioid peptides, neprilysin is another member of this group, in the case of the metallopeptidases and aspartic, the nucleophiles clan or family for example MA, is an activated water molecule. The peptidase domain for members of this family also contains a bacterial member and resembles that of thermolysin the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. Thermolysin complexed with the inhibitor (S)-thiorphan are isomeric thiol-containing inhibitors of endopeptidase EC 24-11 (also called "enkephalinase").

Astacins are a family of multidomain metalloendopeptidases which are either secreted or membrane-anchored. These metallopeptidases belong to the MEROPS peptidase family M12, subfamily M12A. The protein fold of the peptidase domain for members of this family resembles that of thermolysin, the type example for clan MA and the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH.
Membrane dipeptidase (EC 3.4.13.19, renal dipeptidase, dehydropeptidase I (DPH I), dipeptidase, aminodipeptidase, dipeptide hydrolase, dipeptidyl hydrolase, nonspecific dipeptidase, glycosyl-phosphatidylinositol-anchored renal dipeptidase, MBD, MDP, leukotriene D4 hydrolase) is an enzyme. This enzyme catalyses the following chemical reaction
Peptidyl-Lys metalloendopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Peptidyl-Asp metalloendopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin C is an enzyme. This enzyme catalyses the following chemical reaction
Pitrilysin is an enzyme. This enzyme catalyses the following chemical reaction:
Nardilysin is an enzyme. This enzyme catalyses the following chemical reaction
N-glycosyltransferase is an enzyme in prokaryotes which transfers individual hexoses onto asparagine sidechains in substrate proteins, using a nucleotide-bound intermediary, within the cytoplasm. They are distinct from regular N-glycosylating enzymes, which are oligosaccharyltransferases that transfer pre-assembled oligosaccharides. Both enzyme families however target a shared amino acid sequence asparagine—-any amino acid except proline—serine or threonine (N–x–S/T), with some variations.
References
- ↑ Tarentino, A.L.; Quinones, G.; Grimwood, B.G.; Hauer, C.R.; Plummer, T.H. Jr. (1995). "Molecular cloning and sequence analysis of flavastacin: an O-glycosylated prokaryotic zinc metalloendopeptidase". Arch. Biochem. Biophys. 319: 281–285. doi:10.1006/abbi.1995.1293. PMID 7771796.
- ↑ Pralow A, Hoffmann M, Nguyen-Khuong T, Rapp E, Reichl U (2017). "Improvement of the glycoproteomic toolbox with the discovery of a unique C-terminal cleavage specificity of flavastacin for N-glycosylated asparagine". Sci Rep. 7 (1): 11419. doi:10.1038/s41598-017-11668-1. PMC 5595805 . PMID 28900186.