West Nile fever is an infection by the West Nile virus which is typically spread by mosquitoes. In about 80% of infections people have few or no symptoms. About 20% of people develop a fever, headache, vomiting, or a rash. In less than 1% of people, encephalitis or meningitis occurs, with associated neck stiffness, confusion, or seizures. Recovery may take weeks to months. The risk of death among those in whom the nervous system is affected is about 10%.

Flavivirus is a genus of viruses in the family Flaviviridae. This genus includes the West Nile virus, dengue virus, tick-borne encephalitis virus, yellow fever virus, Zika virus and several other viruses which may cause encephalitis, as well as insect-specific flaviviruses (ISFs) such as cell fusing agent virus (CFAV), Palm Creek virus (PCV), and Parramatta River virus (PaRV).
La Crosse encephalitis is an encephalitis caused by an arbovirus which has a mosquito vector.

Bunyavirales is an order of negative-sense single-stranded RNA viruses. It is the only order in the class Ellioviricetes. It was formerly known as Bunyaviridae family of viruses. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

Japanese encephalitis (JE) is an infection of the brain caused by the Japanese encephalitis virus (JEV). While most infections result in little or no symptoms, occasional inflammation of the brain occurs. In these cases symptoms may include headache, vomiting, fever, confusion, and seizures. This occurs about 5 to 15 days after infection.
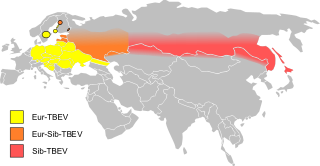
Tick-borne encephalitis (TBE) is a viral infectious disease involving the central nervous system. The disease most often manifests as meningitis, encephalitis, or meningoencephalitis. Long-lasting or permanent neuropsychiatric consequences are observed in 10 to 20% of infected patients.

Oropouche fever is a tropical viral infection transmitted by biting midges and mosquitoes from the blood of sloths to humans. This disease is named after the region where it was first discovered and isolated at the Trinidad Regional Virus Laboratory in 1955 by the Oropouche River in Trinidad and Tobago. Oropouche fever is caused by a specific arbovirus, the Oropouche virus (OROV), of the Bunyaviridae family.

In biology and immunology, an Alphavirus belongs to group IV of the Baltimore classification of the Togaviridae family of viruses, according to the system of classification based on viral genome composition introduced by David Baltimore in 1971. Alphaviruses, like all other group IV viruses, have a positive sense, single-stranded RNA genome. There are thirty alphaviruses able to infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses as well as invertebrates. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.
California encephalitis orthobunyavirus or California encephalitis virus was discovered in Kern County, California and causes encephalitis in humans. Encephalitis is an acute inflammation of the brain that can cause minor symptoms, such as headaches, to more severe symptoms such as seizures. Mosquitoes serve as its carrier and for this reason this virus is known as an arbovirus.
Mosquito-borne diseases or mosquito-borne illnesses are diseases caused by bacteria, viruses or parasites transmitted by mosquitoes. They can transmit disease without being affected themselves. Nearly 700 million people get a mosquito-borne illness each year resulting in over one million deaths.

Zika virus (ZIKV) is a member of the virus family Flaviviridae. It is spread by daytime-active Aedes mosquitoes, such as A. aegypti and A. albopictus. Its name comes from the Ziika Forest of Uganda, where the virus was first isolated in 1947. Zika virus is related to the dengue, yellow fever, Japanese encephalitis, and West Nile viruses. Since the 1950s, it has been known to occur within a narrow equatorial belt from Africa to Asia. From 2007 to 2016, the virus spread eastward, across the Pacific Ocean to the Americas, leading to the 2015–16 Zika virus epidemic.
Langat virus (LGTV) is a virus of the genus Flavivirus. The virus was first isolated in Malaysia in 1956 from a hard tick of the Ixodes genus. This virus is antigenically related to Omsk hemorrhagic fever virus, Kyasanur forest disease virus, Alkhurma virus, Louping ill virus and other viruses of the tick-borne encephalitis virus (TBEV) complex. The Langat virus does not pose a significant epidemiological threat in comparison with TBEV. There are no known cases of human diseases associated with LGTV. The Malaysian strain is naturally attenuated and induces neutralizing antibodies to tick-borne encephalitis virus (TBEV) and protection against other TBEV complex viruses in animals.
Usutu virus (USUV) first identified in South Africa in 1959, is an emerging zoonotic arbovirus of concern because of its pathogenicity to humans and its similarity in ecology with other emerging arboviruses such as West Nile Virus. USUV is a flavivirus belonging to the Japanese encephalitis complex.
Tahyna virus ("TAHV") is a viral pathogen of humans classified in the California encephalitis virus (CEV) serogroup of the Orthobunyavirus family in the order Bunyavirales, which is endemic to Europe, Asia, Africa and possibly China.
Cache Valley virus (CVV) is a member of the order Bunyavirales, genus Orthobunyavirus, and serogroup Bunyamwera, which was first isolated in 1956 from Culiseta inornata mosquitos collected in Utah’s Cache Valley. CVV is an enveloped arbovirus, nominally 80–120 nm in diameter, whose genome is composed of three single-stranded, negative-sense RNA segments. The large segment of related bunyaviruses is approximately 6800 bases in length and encodes a probable viral polymerase. The middle CVV segment has a 4463-nucleotide sequence and the smallest segment encodes for the nucleocapsid, and a second non-structural protein. CVV has been known to cause outbreaks of spontaneous abortion and congenital malformations in ruminants such as sheep and cattle. CVV rarely infects humans, but when they are infected it has caused encephalitis and multiorgan failure.
Tensaw virus is a virus in the genus Orthobunyavirus of the Bunyamwera arbovirus group, order Bunyavirales. It is named for the river bordering the area in south Alabama where the prototype strain was discovered. It is abbreviated TEN, TENV, and TSV in the scientific literature.

West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, specifically from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. West Nile virus is primarily transmitted by mosquitoes, mostly species of the genus Culex. The primary hosts of WNV are birds, so that the virus remains within a "bird–mosquito–bird" transmission cycle.
Maguari virus, abbreviated MAGV, is a negative-sense, single-stranded RNA virus in the Bunyavirales order, genus Orthobunyavirus, Bunyamwera serogroup, that has been shown to be capable of causing human disease. MAGV is related to Cache Valley virus and Tensaw virus.