
Lassa fever, also known as Lassa hemorrhagic fever, is a type of viral hemorrhagic fever caused by the Lassa virus. Many of those infected by the virus do not develop symptoms. When symptoms occur they typically include fever, weakness, headaches, vomiting, and muscle pains. Less commonly there may be bleeding from the mouth or gastrointestinal tract. The risk of death once infected is about one percent and frequently occurs within two weeks of the onset of symptoms. Of those who survive, about a quarter have hearing loss, which improves within three months in about half of these cases.

Orthohantavirus is a genus of single-stranded, enveloped, negative-sense RNA viruses in the family Hantaviridae within the order Bunyavirales. Members of this genus may be called orthohantaviruses or simply hantaviruses.
Bolivian hemorrhagic fever (BHF), also known as black typhus or Ordog Fever, is a hemorrhagic fever and zoonotic infectious disease originating in Bolivia after infection by Machupo mammarenavirus.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

Lassa mammarenavirus (LASV) is an arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa mammarenavirus is an emerging virus and a select agent, requiring Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease, mostly in boa constrictors. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.

Viral hemorrhagic fevers (VHFs) are a diverse group of animal and human illnesses. VHFs may be caused by five distinct families of RNA viruses: the families Filoviridae, Flaviviridae, Rhabdoviridae, and several member families of the Bunyavirales order such as Arenaviridae, and Hantaviridae. All types of VHF are characterized by fever and bleeding disorders and all can progress to high fever, shock and death in many cases. Some of the VHF agents cause relatively mild illnesses, such as the Scandinavian nephropathia epidemica, while others, such as Ebola virus, can cause severe, life-threatening disease.
Omsk hemorrhagic fever is a viral hemorrhagic fever caused by a Flavivirus.
Seoul orthohantavirus (SEOV) is a member of the genus Orthohantavirus of rodent-borne viruses, and is one of the four hantaviruses that are known to cause Hantavirus hemorrhagic fever with renal syndrome (HFRS). It is an Old World hantavirus; a negative sense, single-stranded, tri-segmented RNA virus.

Mammarenavirus juninense, better known as the Junin virus or Junín virus (JUNV), is an arenavirus in the Mammarenavirus genus that causes Argentine hemorrhagic fever (AHF). The virus took its original name from the city of Junín, around which the first cases of infection were reported, in 1958.

Chapare mammarenavirus or Chapare virus is a virus from the family Arenaviridae which causes a hemorrhagic fever in humans known as Chapare hemorrhagic fever. It was first described after an outbreak of a novel zoonotic mammarenavirus infection occurred in the village of Samuzabeti, Chapare Province, Bolivia, in January 2003. A small number of people were infected and one person died.
A robovirus is a zoonotic virus that is transmitted by a rodent vector.
Lujo is a bisegmented RNA virus—a member of the family Arenaviridae—and a known cause of viral hemorrhagic fever (VHF) in humans. Its name was suggested by the Special Pathogens Unit of the National Institute for Communicable Diseases of the National Health Laboratory Service (NICD-NHLS) by using the first two letters of the names of the cities involved in the 2008 outbreak of the disease, Lusaka (Zambia) and Johannesburg. It is the second pathogenic Arenavirus to be described from the African continent—the first being Lassa virus—and since 2012 has been classed as a "Select Agent" under U.S. law.
Brazilian hemorrhagic fever (BzHF) is an infectious disease caused by Brazilian mammarenavirus, an arenavirus. Brazilian mammarenavirus is one of the arenaviruses from South America to cause hemorrhagic fever. It shares a common progenitor with Argentinian mammarenavirus, Machupo mammarenavirus, Tacaribe mammarenavirus, and Guanarito mammarenavirus. It is an enveloped RNA virus and is highly infectious and lethal. Very little is known about this disease, but it is thought to be transmitted by the excreta of rodents. This virus has also been implicated as a means for bioterrorism, as it can be spread through aerosols.
Caño Delgadito orthohantavirus (CADV) is a hantavirus present in Venezuela. Its natural reservoir is Alston's cotton rat. Transmission among cotton rats appears to be horizontal. While human disease caused by CADV has not yet been identified, it has been isolated from oropharyngeal swabs and urine of infected cotton rats, indicating that it may be infectious to humans in the same manner as other hantaviruses, via inhalation of aerosolized droplets of saliva, respiratory secretions, or urine. CADV was discovered in the 1990s from rodent species in the Llanos in Venezuela.
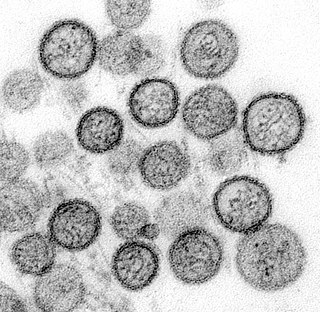
Hantavirus hemorrhagic fever with renal syndrome (HFRS) is a group of clinically similar illnesses caused by species of hantaviruses. It is also known as Korean hemorrhagic fever and epidemic hemorrhagic fever. It is found in Europe, Asia, and Africa. The species that cause HFRS include Hantaan orthohantavirus, Dobrava-Belgrade orthohantavirus, Saaremaa virus, Seoul orthohantavirus, Puumala orthohantavirus and other orthohantaviruses. Of these species, Hantaan River virus and Dobrava-Belgrade virus cause the most severe form of the syndrome and have the highest morbidity rates. When caused by the Puumala virus, it is also called nephropathia epidemica. This infection is known as sorkfeber in Swedish, myyräkuume in Finnish, and musepest in Norwegian.
Flexal mammarenavirus is a mammarenavirus: an arenavirus with a mammalian host. It was first found in semiaquatic rodents of the genus Oryzomys in tropical forest in the Pará area of Brazil.
Whitewater Arroyo mammarenavirus (WWAV) is a zoonotic Arenavirus associated with hemorrhagic fever with liver failure.
Morogoro virus is an East African arenavirus infecting the multimammate mouse. The virus is genetically closely related to Lassa virus, known to cause Lassa fever in humans. Morogoro virus, however, does not seem to infect humans. Transmission of Morogoro virus between mice is assumed to occur via direct and indirect contact. Infected animals pass a latent period of 7 days and subsequently shed the virus for about 30 days, after which they recover and develop lifelong antibodies. Transmission may also be possible from infected mothers to offspring and through sexual contact, as this has been suggested for other arenaviruses.
Mopeia mammarenavirus (MOPV) is a species of virus in the genus Mammarenavirus. It was initially isolated from the Mastomys natalensis mouse in the East African country of Mozambique in 1977. It is of the "Old World" Arenavirus lineage and is closely related to Lassa mammarenavirus, sharing 75% of its amino acid sequence.








