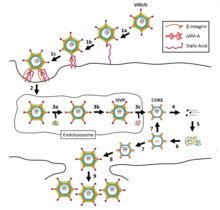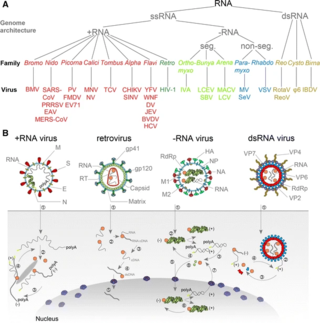
An RNA virus is a virus—other than a retrovirus—that has ribonucleic acid (RNA) as its genetic material. The nucleic acid is usually single-stranded RNA (ssRNA) but it may be double-stranded (dsRNA). Notable human diseases caused by RNA viruses include the common cold, influenza, SARS, MERS, Covid-19, Dengue Virus, hepatitis C, hepatitis E, West Nile fever, Ebola virus disease, rabies, polio, mumps, and measles.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

Orbivirus is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Sedoreovirinae. Unlike other reoviruses, orbiviruses are arboviruses. They can infect and replicate within a wide range of arthropod and vertebrate hosts. Orbiviruses are named after their characteristic doughnut-shaped capsomers.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Orthoreovirus is a genus of viruses, in the family Reoviridae, in the subfamily Spinareovirinae. Vertebrates serve as natural hosts. There are ten species in this genus. Diseases associated with this genus include mild upper respiratory tract disease, gastroenteritis, and biliary atresia. Mammalian orthoreovirus 3 induces cell death preferentially in transformed cells and therefore displays inherent oncolytic properties.
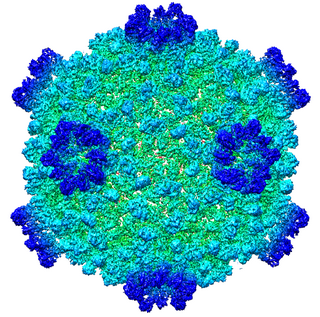
Cypovirus, short for cytoplasmic polyhedrosis virus, is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Spinareovirinae. Cypoviruses have only been isolated from insects. Diseases associated with this genus include chronic diarrhoea and pale blue iridescence in the guts of larvae. Sixteen species are placed in this genus.

The golden shiner virus is an aquatic virus that infects a bait fish known as the golden shiner and to a lesser extent, aquatic animals like crustaceans and molluscs. About 6 virus species have been identified in this genus since the late 1970s. It causes death through a hemorrhagic shock. Symptoms include bleeding from the back eyes and the head. The virus is 70 nm in diameter and replicates best at 20-30 degrees Celsius. The virus has properties similar to those of the pancreatic necrosis virus. This could mean that golden shiners are more susceptible in the summer.

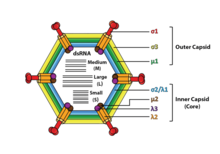
Double-stranded RNA viruses are a polyphyletic group of viruses that have double-stranded genomes made of ribonucleic acid. The double-stranded genome is used as a template by the viral RNA-dependent RNA polymerase (RdRp) to transcribe a positive-strand RNA functioning as messenger RNA (mRNA) for the host cell's ribosomes, which translate it into viral proteins. The positive-strand RNA can also be replicated by the RdRp to create a new double-stranded viral genome.

Phytoreovirus is a genus of viruses, in the family Reoviridae, in the subfamily Sedoreovirinae. They are non-turreted reoviruses that are major agricultural pathogens, particularly in Asia. Oryza sativa for RDV and RGDV, dicotyledonous for WTV, and leafhoppers serve as natural hosts. There are three species in this genus. Diseases associated with this genus include: WTV: galls (tumor). RDV: dwarf disease of rice. RGDV: dwarfing, stunting, and galls.

Corticovirus is a genus of viruses in the family Corticoviridae. Corticoviruses are bacteriophages; that is, their natural hosts are bacteria. The genus contains two species. The name is derived from Latin cortex, corticis. However, prophages closely related to PM2 are abundant in the genomes of aquatic bacteria, suggesting that the ecological importance of corticoviruses might be underestimated. Bacteriophage PM2 was first described in 1968 after isolation from seawater sampled from the coast of Chile.
Avian orthoreovirus, also known as avian reovirus, is an orthoreovirus from the Reoviridae family. Infection causes arthritis and tenosynovitis in poultry. It can also cause respiratory disease.
Xi River virus (XRV) is a putative novel bat virus in the genus Orthoreovirus isolated from fruit bats in Guangdong Province in southern China. It is the first bat reovirus isolated in China.

Sedoreovirinae is a subfamily of the Reoviridae family of viruses. Viruses in this subfamily are distinguished by the absence of a turreted protein on the inner capsid to produce a smooth surface.
Epizootic hemorrhagic disease virus, often abbreviated to EHDV, is a species of the genus Orbivirus, a member of the family Reoviridae. It is the causative agent of epizootic hemorrhagic disease, an acute, infectious, and often fatal disease of wild ruminants. In North America, the most severely affected ruminant is the white-tailed deer, although it may also infect mule deer, black-tailed deer, elk, bighorn sheep, and pronghorn antelope. It is often mistakenly referred to as “bluetongue virus” (BTV), another Orbivirus that like EHDV causes the host to develop a characteristic blue tongue due to systemic hemorrhaging and lack of oxygen in the blood. Despite showing clinical similarities, these two viruses are genetically distinct.
Mimoreovirus is a genus of viruses, in the family Reoviridae, in the subfamily Sedoreovirinae. The only isolate infects the marine photosynthetic protist Micromonas pusilla, a prasinophyte. There is only one species in this genus: Micromonas pusilla reovirus.
Triatoma virus (TrV) is a virus belonging to the insect virus family Dicistroviridae. Within this family, there are currently 3 genera and 15 species of virus. Triatoma virus belongs to the genus Cripavirus. It is non-enveloped and its genetic material is positive-sense, single-stranded RNA. The natural hosts of triatoma virus are invertebrates. TrV is a known pathogen to Triatoma infestans, the major vector of Chagas disease in Argentina which makes triatoma virus a major candidate for biological vector control as opposed to chemical insecticides. Triatoma virus was first discovered in 1984 when a survey of pathogens of triatomes was conducted in the hopes of finding potential biological control methods for T. infestans.
Umatilla virus(UMAV) is a dsRNA virus in the family Reoviridae, subfamily Sedoreovirinae, and the genus Orbivirus. This arbovirus was first isolated in 1969 in Umatilla County, Oregon in a group of Culex pipiens mosquitoes. The viral host is the Passer domesticus bird with the vectors being Culex mosquitoes.
Mammalian orthoreovirus (MRV) is a double-stranded RNA virus. It is a part of the family Reoviridae, as well as the subfamily Spinareovirinae. As seen in the name, the Mammalian Ortheoreovirus infects numerous mammalian species and vertebrates which serve as natural hosts. Some diseases that occur as a result of this virus or are associated with this virus include mild upper respiratory illness, and gastrointestinal illness. Examples of these are: upper respiratory tract syndromes, gastroenteritis, biliary atresia, obstructive hydrocephalus, jaundice, alopecia, conjunctivitis, and ‘oily hair’ associated with steatorrhea.

Astroviridae is a family of non-enveloped ssRNA viruses that cause infections in different animals. The family name is derived from the Greek word astron ("star") referring to the star-like appearance of spikes projecting from the surface of these small unenveloped viruses. Astroviruses were initially identified in humans but have since been isolated from other mammals and birds. This family of viruses consists of two genera, Avastrovirus (AAstV) and Mamastrovirus (MAstV). Astroviruses most frequently cause infection of the gastrointestinal tract but in some animals they may result in encephalitis, hepatitis (avian) and nephritis (avian).
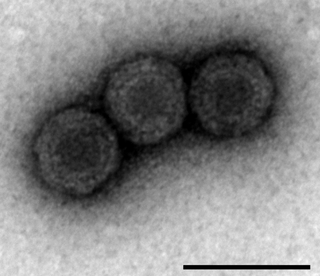
Piscine orthoreovirus (PRV) is a species in the genus Orthoreovirus that infects fish exclusively, PRV was first discovered in 2010 in farmed Atlantic salmon exhibiting Heart and Skeletal Muscle Inflammation (HSMI) and has been found present at higher concentration in fish with various diseases. These diseases include HSMI, jaundice syndrome, proliferative darkening syndrome and erythrocytic body inclusion syndrome. PRV is thought to mainly affect aquacultured and maricultured fish stocks, and recent research has been focused around the susceptibility of wild stock. However, whether PRV is virulent with respect to HSMI remains a topic of debate. PRV has been in the public eye mostly due to a potential linkage to farmed Atlantic Salmon exhibiting HSMI. Public concern has been raised regarding the possibility of open ocean-net farms transmitting PRV to wild salmon populations and being a factor in declining populations. PRV has not been confirmed to be pathogenic in wild salmon stocks.