Related Research Articles

Bradykinin (BK) (Greek brady-, slow; -kinin, kīn(eîn) to move) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin consists of nine amino acids, and is a physiologically and pharmacologically active peptide of the kinin group of proteins.
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal, an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide.
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein has no known paralogue, while tissue kallikrein-related peptidases (KLKs) encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation.
Membrane-type matrix metalloproteinase-1 is an enzyme. This enzyme catalyses the following chemical reaction

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.

In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:
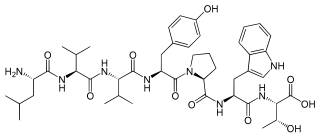
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.
Pancreatic elastase II is an enzyme. This enzyme catalyses the following chemical reaction
Snake venom factor V activator is an enzyme. This enzyme catalyses the following chemical reaction
Bothropasin is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin B is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin C is an enzyme. This enzyme catalyses the following chemical reaction
Ruberlysin is an enzyme. This enzyme catalyses the following chemical reaction
Bothrolysin is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin II is an enzyme. This enzyme catalyses the following chemical reaction
Mucrolysin is an enzyme. This enzyme catalyses the following chemical reaction
Fibrolase is an enzyme. This enzyme catalyses the following chemical reaction
Jararhagin is an enzyme. This enzyme catalyses the following chemical reaction

The sedolisin family of peptidases are a family of serine proteases structurally related to the subtilisin (S8) family. Well-known members of this family include sedolisin ("pseudomonalisin") found in Pseudomonas bacteria, xanthomonalisin ("sedolisin-B"), physarolisin as well as animal tripeptidyl peptidase I. It is also known as sedolysin or serine-carboxyl peptidase. This group of enzymes contains a variation on the catalytic triad: unlike S8 which uses Ser-His-Asp, this group runs on Ser-Glu-Asp, with an additional acidic residue Asp in the oxyanion hole.
References
- ↑ Wagner FW, Spiekerman AM, Prescott JM (September 1968). "Leucostoma peptidase A. Isolation and physical properties". The Journal of Biological Chemistry. 243 (17): 4486–93. PMID 5684005.
- ↑ Spiekerman AM, Fredericks KK, Wagner FW, Prescott JM (February 1973). "Leucostoma peptidase A: a metalloprotease from snake venom". Biochimica et Biophysica Acta (BBA) - Enzymology. 293 (2): 464–75. doi:10.1016/0005-2744(73)90353-7. PMID 4711816.