Related Research Articles
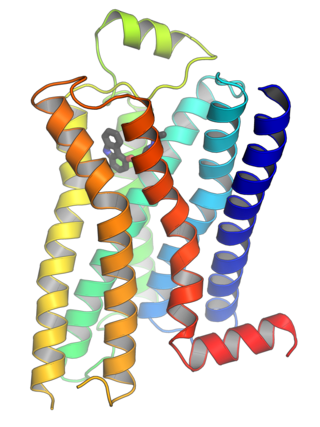
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.
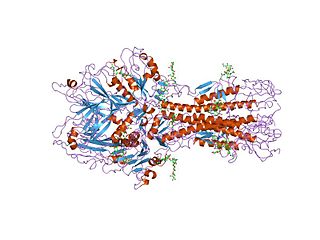
Hemagglutinin esterase (HEs) is a glycoprotein that certain enveloped viruses possess and use as an invading mechanism. HEs helps in the attachment and destruction of certain sialic acid receptors that are found on the host cell surface. Viruses that possess HEs include influenza C virus, toroviruses, and coronaviruses of the subgenus Embecovirus. HEs is a dimer transmembrane protein consisting of two monomers, each monomer is made of three domains. The three domains are: membrane fusion, esterase, and receptor binding domains.

Oligo-1,6-glucosidase is a glucosidase enzyme located on the brush border of the small intestine, which catalyses the following reaction:

In molecular biology, CD18 is an integrin beta chain protein that is encoded by the ITGB2 gene in humans. Upon binding with one of a number of alpha chains, CD18 is capable of forming multiple heterodimers, which play significant roles in cellular adhesion and cell surface signaling, as well as important roles in immune responses. CD18 also exists in soluble, ligand binding forms. Deficiencies in CD18 expression can lead to adhesion defects in circulating white blood cells in humans, reducing the immune system's ability to fight off foreign invaders.

Gastric lipase, also known as LIPF, is an enzymatic protein that, in humans, is encoded by the LIPF gene.

Caveolin-1 is a protein that in humans is encoded by the CAV1 gene.

AP-1 complex subunit gamma-1 is a protein that in humans is encoded by the AP1G1 gene.

Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform is an enzyme regulator that in humans is encoded by the PPP2R2A gene.

Hexosaminidase A (alpha polypeptide), also known as HEXA, is an enzyme that in humans is encoded by the HEXA gene, located on the 15th chromosome.

Integrin beta-7 is an integrin protein that in humans is encoded by the ITGB7 gene. It can pair with ITGA4 (CD49d) to form the heterodimeric integrin receptor α4β7, or with ITGAE (CD103) to form αEβ7.

Potassium voltage-gated channel subfamily A member 2 also known as Kv1.2 is a protein that in humans is encoded by the KCNA2 gene.

Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A beta isoform is an enzyme that in humans is encoded by the PPP2R1B gene.

ATP synthase F1 subunit alpha, mitochondrial is an enzyme that in humans is encoded by the ATP5F1A gene.

Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta is the beta subunit of the protein complex PDE6 that is encoded by the PDE6B gene. PDE6 is crucial in transmission and amplification of visual signal. The existence of this beta subunit is essential for normal PDE6 functioning. Mutations in this subunit are responsible for retinal degeneration such as retinitis pigmentosa or congenital stationary night blindness.

Meprin A subunit alpha also known as endopeptidase-2 or PABA peptide hydrolase is the alpha subunit of the meprin A enzyme that in humans is encoded by the MEP1A gene. The MEP1A locus is on chromosome 6p in humans and on chromosome 17 in mice.

Meprin A subunit beta is a protein that in humans is encoded by the MEP1B gene.

Dipeptidase 1 (DPEP1), or renal dipeptidase, is a membrane-bound glycoprotein responsible for hydrolyzing dipeptides. It is found in the microsomal fraction of the porcine kidney cortex. It exists as a disulfide-linked homodimer that is glygosylphosphatidylinositol (GPI)-anchored to the renal brush border of the kidney. The active site on each homodimer is made up of a barrel subunit with binuclear zinc ions that are bridged by the Gly125 side-chain located at the bottom of the barrel.

Disintegrin and metalloproteinase domain-containing protein 2 or Beta-fertilin is an enzyme that in humans is encoded by the ADAM2 gene.

ATP synthase F1 subunit epsilon, mitochondrial is an enzyme that in humans is encoded by the ATP5F1E gene. The protein encoded by ATP5F1E is a subunit of ATP synthase, also known as Complex V. Variations of this gene have been associated with mitochondrial complex V deficiency, nuclear 3 (MC5DN3) and Papillary Thyroid Cancer.
MAM domain is an evolutionary conserved protein domain. It is an extracellular domain found in many receptors.
References
- ↑ Kounnas MZ, Wolz RL, Gorbea CM, Bond JS (September 1991). "Meprin-A and -B. Cell surface endopeptidases of the mouse kidney". The Journal of Biological Chemistry. 266 (26): 17350–7. PMID 1894622.
- ↑ Gorbea CM, Marchand P, Jiang W, Copeland NG, Gilbert DJ, Jenkins NA, Bond JS (October 1993). "Cloning, expression, and chromosomal localization of the mouse meprin beta subunit". The Journal of Biological Chemistry. 268 (28): 21035–43. PMID 8407940.
- ↑ Johnson GD, Hersh LB (March 1994). "Expression of meprin subunit precursors. Membrane anchoring through the beta subunit and mechanism of zymogen activation". The Journal of Biological Chemistry. 269 (10): 7682–8. PMID 7510289.
- ↑ Wolz RL, Bond JS (1995). Meprins A and B. Methods in Enzymology. Vol. 248. pp. 325–45. doi:10.1016/0076-6879(95)48022-6. PMID 7674930.
- 1 2 Ishmael, Faoud T.; Shier, Vincent K.; Ishmael, Susan S.; Bond, Judith S. (February 2005). "Intersubunit and Domain Interactions of the Meprin B Metalloproteinase DISULFIDE BONDS AND PROTEIN-PROTEIN INTERACTION IN THE MAM AND TRAF DOMAINS". Journal of Biological Chemistry. 280: 13895–13901.