Classification
Alkyl and aryl monolithium compounds
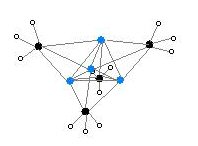

 Various depictions of the methyl lithium tetramer. Colour code: Li- purple C- black H- white
Various depictions of the methyl lithium tetramer. Colour code: Li- purple C- black H- white
Alkyl and aryl monolithium are illustrated by their simplest members, methyl lithium and phenyl lithium. These useful reagents have complicated structures owing to the tendency of the C-Li unit to aggregate, forming clusters. [2] These compounds are prepared by metal-halogen exchange, which entails stirring solutions of the alkyl halide with lithium metal:
- CH3Br + Li → CH3Li + LiBr
Lithium-reduced polycyclic aromatics

Naphthalene, anthracene, and related polycyclic aromatic compounds are reduced by lithium to give Li+ salts of their radical anions: [3]
- C10H8 + Li → Li+C10H−8
Lithium naphthalene ( Li +[ C 10 H 8]−) is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry.
Dilithio compounds
These compounds, which have two C-Li bonds can be classified into two distinct groups. Simplest but rare is dilithiomethanide (CH2Li2) and its many derivatives. [4] Some derivatives have even been characterized by X-ray crystallography. [5]
The more common dilithiated organic compounds the Li atoms are bound to separate carbon atoms. 1,4-dilithiobutane (LiCH2CH2CH2CH2Li) would be an example.
Dilithioacetylide (Li2C2, again is not a salt, but adopts a distorted anti-fluorite crystal structure, similar to that of rubidium peroxide (Rb2O2). [6]
Tetralithio compounds
Tetralithiomethane has attracted attention mainly for theoretical reasons. Its structure remains uncertain, but some speculation posited planar carbon.