A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.

Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine, as well as of a large number of secondary metabolites of the shikimate pathway.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

In enzymology, a shikimate dehydrogenase (EC 1.1.1.25) is an enzyme that catalyzes the chemical reaction
In enzymology, an aryl-alcohol dehydrogenase (EC 1.1.1.90) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydroxyphenylpyruvate reductase (EC 1.1.1.237) is an enzyme that catalyzes the chemical reaction
In enzymology, a quinate dehydrogenase (EC 1.1.1.24) is an enzyme that catalyzes the chemical reaction
In enzymology, an arogenate dehydrogenase (EC 1.3.1.43) is an enzyme that catalyzes the chemical reaction
In enzymology, an arogenate dehydrogenase [NAD(P)+] (EC 1.3.1.79) is an enzyme that catalyzes the chemical reaction
In enzymology, an arogenate dehydrogenase (NADP+) (EC 1.3.1.78) is an enzyme that catalyzes the chemical reaction
In enzymology, a prephenate dehydrogenase (NADP+) (EC 1.3.1.13) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-hydroxyphenylacetaldehyde dehydrogenase (EC 1.2.1.53) is an enzyme that catalyzes the chemical reaction
In enzymology, a 5-carboxymethyl-2-hydroxymuconic-semialdehyde dehydrogenase (EC 1.2.1.60) is an enzyme that catalyzes the chemical reaction
In enzymology, a succinate-semialdehyde dehydrogenase [NAD(P)+] (EC 1.2.1.16) is an enzyme that catalyzes the chemical reaction

In enzymology, chorismate mutase is an enzyme that catalyzes the chemical reaction for the conversion of chorismate to prephenate in the pathway to the production of phenylalanine and tyrosine, also known as the shikimate pathway. Hence, this enzyme has one substrate, chorismate, and one product, prephenate. Chorismate mutase is found at a branch point in the pathway. The enzyme channels the substrate, chorismate to the biosynthesis of tyrosine and phenylalanine and away from tryptophan. Its role in maintaining the balance of these aromatic amino acids in the cell is vital. This is the single known example of a naturally occurring enzyme catalyzing a pericyclic reaction. Chorismate mutase is only found in fungi, bacteria, and higher plants. Some varieties of this protein may use the morpheein model of allosteric regulation.
The enzyme prephenate dehydratase (EC 4.2.1.51) catalyzes the chemical reaction
In enzymology, glutamate-prephenate aminotransferase is an enzyme that catalyzes the chemical reaction

Shikimate kinase (EC 2.7.1.71) is an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate. This reaction is the fifth step of the shikimate pathway, which is used by plants and bacteria to synthesize the common precursor of aromatic amino acids and secondary metabolites. The systematic name of this enzyme class is ATP:shikimate 3-phosphotransferase. Other names in common use include shikimate kinase (phosphorylating), and shikimate kinase II.
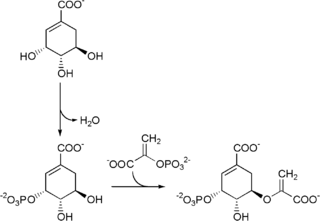
The shikimate pathway is a seven-step metabolic pathway used by bacteria, archaea, fungi, algae, some protozoans, and plants for the biosynthesis of folates and aromatic amino acids. This pathway is not found in animal cells.









