Related Research Articles

Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane.

Legionella pneumophila is a thin, aerobic, pleomorphic, flagellated, non-spore-forming, Gram-negative bacterium of the genus Legionella. L. pneumophila is the primary human pathogenic bacterium in this group and is the causative agent of Legionnaires' disease, also known as legionellosis.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes.
The oxidase test is used to determine if an organism possesses the cytochrome c oxidase enzyme. The test is used as an aid for the differentiation of Neisseria, Moraxella, Campylobacter and Pasteurella species. It is also used to differentiate pseudomonads from related species.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.
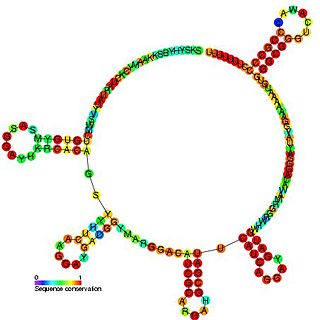
The PrrB/RsmZ RNA family are a group of related non-coding RNA molecules found in bacteria. PrrB RNA is able to phenotypically complement gacS and gacA mutants and is itself regulated by the GacS-GacA two-component signal transduction system. Inactivation of the prrB gene in Pseudomonas fluorescens F113 resulted in a significant reduction of 2, 4-diacetylphloroglucinol (Phl) and hydrogen cyanide (HCN) production, while increased metabolite production was observed when prrB was overexpressed. The prrB gene sequence contains a number of imperfect repeats of the consensus sequence 5′-AGGA-3′, and sequence analysis predicted a complex secondary structure featuring multiple putative stem-loops with the consensus sequences predominantly positioned at the single-stranded regions at the ends of the stem-loops. This structure is similar to the CsrB and RsmB regulatory RNAs, suggesting this RNA also interacts with a CsrA-like protein.

The rsmY RNA family is a set of related non-coding RNA genes, that like RsmZ, is regulated by the GacS/GacA signal transduction system in the plant-beneficial soil bacterium and biocontrol model organism Pseudomonas fluorescens CHA0. GacA/GacS target genes are translationally repressed by the small RNA binding protein RsmA. RsmY and RsmZ RNAs bind RsmA to relieve this repression and so enhance secondary metabolism and biocontrol traits.

Adenylylation, more commonly known as AMPylation, is a process in which an adenosine monophosphate (AMP) molecule is covalently attached to the amino acid side chain of a protein. This covalent addition of AMP to a hydroxyl side chain of the protein is a post-translational modification. Adenylylation involves a phosphodiester bond between a hydroxyl group of the molecule undergoing adenylylation, and the phosphate group of the adenosine monophosphate nucleotide. Enzymes that are capable of catalyzing this process are called AMPylators.
Autoinducers are signaling molecules that are produced in response to changes in cell-population density. As the density of quorum sensing bacterial cells increases so does the concentration of the autoinducer. Detection of signal molecules by bacteria acts as stimulation which leads to altered gene expression once the minimal threshold is reached. Quorum sensing is a phenomenon that allows both Gram-negative and Gram-positive bacteria to sense one another and to regulate a wide variety of physiological activities. Such activities include symbiosis, virulence, motility, antibiotic production, and biofilm formation. Autoinducers come in a number of different forms depending on the species, but the effect that they have is similar in many cases. Autoinducers allow bacteria to communicate both within and between different species. This communication alters gene expression and allows bacteria to mount coordinated responses to their environments, in a manner that is comparable to behavior and signaling in higher organisms. Not surprisingly, it has been suggested that quorum sensing may have been an important evolutionary milestone that ultimately gave rise to multicellular life forms.

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction

Pyocyanin (PCN−) is one of the many toxic compounds produced and secreted by the Gram negative bacterium Pseudomonas aeruginosa. Pyocyanin is a blue secondary metabolite, turning red below pH 4.9, with the ability to oxidise and reduce other molecules and therefore kill microbes competing against P. aeruginosa as well as mammalian cells of the lungs which P. aeruginosa has infected during cystic fibrosis. Since pyocyanin is a zwitterion at blood pH, it is easily able to cross the cell membrane. There are three different states in which pyocyanin can exist: oxidized (blue), monovalently reduced (colourless) or divalently reduced (red). Mitochondria play an important role in the cycling of pyocyanin between its redox states. Due to its redox-active properties, pyocyanin generates reactive oxygen species.

Ilomastat (INN), is a broad-spectrum matrix metalloproteinase inhibitor.

Rhamnolipids are a class of glycolipid produced by Pseudomonas aeruginosa, amongst other organisms, frequently cited as bacterial surfactants. They have a glycosyl head group, in this case a rhamnose moiety, and a 3-(hydroxyalkanoyloxy)alkanoic acid (HAA) fatty acid tail, such as 3-hydroxydecanoic acid.
UDP-N-acetyl-2-amino-2-deoxyglucuronate dehydrogenase (EC 1.1.1.335, WlbA, WbpB) is an enzyme with systematic name UDP-N-acetyl-2-amino-2-deoxy-alpha-D-glucuronate:NAD+ 3-oxidoreductase. This enzyme catalyses the following chemical reaction:
Alcohol dehydrogenase (cytochrome c) (EC 1.1.2.8, type I quinoprotein alcohol dehydrogenase, quinoprotein ethanol dehydrogenase) is an enzyme with systematic name alcohol:cytochrome c oxidoreductase. This enzyme catalyses the following chemical reaction
Nitric oxide reductase (cytochrome c) (EC 1.7.2.5) is an enzyme with systematic name nitrous oxide:ferricytochrome-c oxidoreductase. This enzyme catalyses the following chemical reaction
The alpha-D-phosphohexomutases are a large superfamily of enzymes, with members in all three domains of life. Enzymes from this superfamily are ubiquitous in organisms from E. Coli to humans, and catalyze a phosphoryl transfer reaction on a phosphosugar substrate. Four well studied subgroups in the superfamily are:
- Phosphoglucomutase (PGM)
- Phosphoglucomutase/Phosphomannomutase (PGM/PMM)
- Phosphoglucosamine mutase (PNGM)
- Phosphoaceytlglucosamine mutase (PAGM)
UDP-2-acetamido-2-deoxy-ribo-hexuluronate aminotransferase is an enzyme with systematic name UDP-2-acetamido-3-amino-2,3-dideoxy-alpha-D-glucuronate:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction
Serralysin is an enzyme. This enzyme catalyses the following chemical reaction
Everett Peter Greenberg is an American microbiologist. He is the inaugural Eugene and Martha Nester Professor of Microbiology at the Department of Microbiology of the University of Washington School of Medicine. He is best known for his research on quorum sensing, and has received multiple awards for his work.
References
- ↑ Morihara K, Tsuzuki H (1975). "Pseudomonas aeruginosa elastase: affinity chromatography and some properties as a metallo-neutral proteinase". Agric. Biol. Chem. 39: 1123–1128. doi: 10.1271/bbb1961.39.1123 .
- ↑ Nishino N, Powers JC (April 1980). "Pseudomonas aeruginosa elastase. Development of a new substrate, inhibitors, and an affinity ligand". The Journal of Biological Chemistry. 255 (8): 3482–6. PMID 6767718.
- ↑ Dreyfus LA, Iglewski BH (March 1986). "Purification and characterization of an extracellular protease of Legionella pneumophila". Infection and Immunity. 51 (3): 736–43. PMC 260959 . PMID 3512431.
- ↑ Bever RA, Iglewski BH (September 1988). "Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene". Journal of Bacteriology. 170 (9): 4309–14. PMC 211443 . PMID 2842313.
- ↑ Black WJ, Quinn FD, Tompkins LS (May 1990). "Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase". Journal of Bacteriology. 172 (5): 2608–13. PMID 2110146.