
Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Pesticide resistance describes the decreased susceptibility of a pest population to a pesticide that was previously effective at controlling the pest. Pest species evolve pesticide resistance via natural selection: the most resistant specimens survive and pass on their acquired heritable changes traits to their offspring. If a pest has resistance then that will reduce the pesticide's efficacy – efficacy and resistance are inversely related.

Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.

MCPA is a widely used phenoxy herbicide introduced in 1945. It selectively controls broad-leaf weeds in pasture and cereal crops. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.
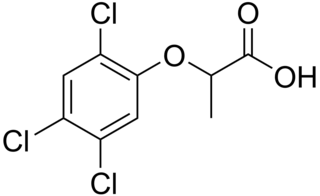
Fenoprop, also called 2,4,5-TP, is the organic compound 2-(2,4,5-trichlorophenoxy)propionic acid. It is a phenoxy herbicide and a plant growth regulator, an analog of 2,4,5-T in which the latter's acetic acid sidechain is replaced with a propionate group (with an extra CH3). The addition of this extra methyl group creates a chiral centre in the molecule and useful biological activity is found only in the (2R)-isomer. The compound's mechanism of action is to mimic the auxin growth hormone indoleacetic acid (IAA). When sprayed on plants it induces rapid, uncontrolled growth. As with 2,4,5-T, fenoprop is toxic to shrubs and trees.

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.
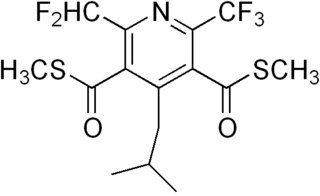
Dithiopyr is a preemergent herbicide for crabgrass control in turf and ornamental grasses. It is effective on 45 grassy and broadleaf weeds. Dithiopyr inhibits root growth of susceptible weeds as well as turf grass and thus should be used only on established turf with a well-developed root system. Its duration of efficacy is approximately 4 months, so lawns should not be reseeded during this time frame following application of the chemical. Dithiopyr acts primarily as a preemergent herbicide but can also be used in early postemergent control of crabgrass.
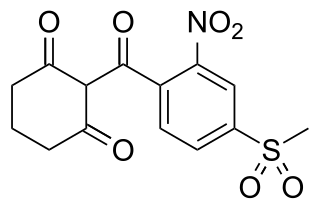
Mesotrione is a selective herbicide used mainly in maize crops. It is a synthetic compound inspired by the natural substance leptospermone found in the bottlebrush tree Callistemon citrinus. It inhibits the enzyme 4-hydroxyphenylpyruvate dioxygenase (HPPD) and is sold under brand names including Callisto and Tenacity. It was first marketed by Syngenta in 2001.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.
Fenpropimorph is a morpholine-derived fungicide used in agriculture, primarily on cereal crops such as wheat. It has been reported to disrupt eukaryotic sterol biosynthesis pathways, notably by inhibiting fungal Δ14 reductases. It has also been reported to inhibit mammalian sterol biosynthesis by affecting lanosterol demethylation. Although used in agriculture for pest management purposes, it has been reported to have a strong adverse effect on sterol biosynthesis in higher-plants by inhibiting the cycloeucalenol-obtusifoliol isomerase. This inhibition was shown to not only alter the lipid composition of the plasma-membrane, but also impact cell division and growth, in plants.
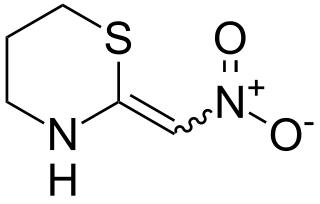
Nithiazine is a nitromethylene neonicotinoid insecticide. It is irritating to the eyes and skin, and is moderately toxic to mammals.

Monochoria korsakowii is a species of annual flowering plant in the water hyacinth family known by several common names, including heartleaf false pickerelweed and oval-leafed pondweed. It is found in lakes, ponds, swamps, and rice paddy fields.

Cyanazine is a herbicide that belongs to the group of triazines. Cyanazine inhibits photosynthesis and is therefore used as a herbicide.

Pyroxasulfone is a pre-emergence herbicide that inhibits the production of very long chain fatty acids in plants. The structure of the existing herbicide thiobencarb served as the basis for development but pyroxasulfone requires a lower dose (100–25 g/ha) and is more stable resulting in longer efficacy. As of 2016 it had been registered for use in Japan, Australia, USA, Canada, Saudi Arabia and South Africa and was used on crops including maize, soybean, wheat and cotton. In 2015 it was applied to over 6 million hectares of land. Pyroxasulfone is from a novel chemical class but has a similar mode of action to acetamide herbicides such as metolachlor, acetochlor and dimethenamid. It is mainly used to control annual grasses but is also effective against broadleaf weeds including lambsquarters, pigweed and waterhemp and black nightshade

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO) which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

Fluazifop is the common name used by the ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Indaziflam is a preemergent herbicide especially for grass control in tree and bush crops.

Aclonifen is a diphenyl ether herbicide which has been used in agriculture since the 1980s. Its mode of action has been uncertain, with evidence suggesting it might interfere with carotenoid biosynthesis or inhibit the enzyme protoporphyrinogen oxidase (PPO). Both mechanisms could result in the observed whole-plant effect of bleaching and the compound includes chemical features that are known to result in PPO effects, as seen with acifluorfen, for example. In 2020, further research revealed that aclonifen has a different and novel mode of action, targeting solanesyl diphosphate synthase which would also cause bleaching.

Chlorsulfuron is an ALS inhibitor herbicide, and is a sulfonylurea compound. It was discovered by George Levitt in February 1976 while working at DuPont, which was the patent assignee.



















