 | |
| Names | |
|---|---|
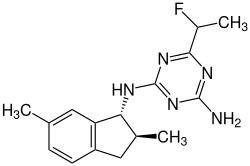
| IUPAC name 2-N-[(1R,2S)-2,6-dimethyl-2,3-dihydro-1H-inden-1-yl]-6-(1-fluoroethyl)-1,3,5-triazine-2,4-diamine [1] | |
| Identifiers | |
3D model (JSmol) | |
| 20920435 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.216.692 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties [2] | |
| C16H20FN5 | |
| Molar mass | 301.369 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 183 °C (361 °F; 456 K) |
| 2.8 mg/L (20 °C) | |
| log P | 2.8 |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H373, H410 | |
| P260, P273, P314, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Indaziflam is a preemergent herbicide used especially for grass control in tree and bush crops.