Muscle spindles are stretch receptors within the body of a skeletal muscle that primarily detect changes in the length of the muscle. They convey length information to the central nervous system via afferent nerve fibers. This information can be processed by the brain as proprioception. The responses of muscle spindles to changes in length also play an important role in regulating the contraction of muscles, for example, by activating motor neurons via the stretch reflex to resist muscle stretch.

A gait is a manner of limb movements made during locomotion. Human gaits are the various ways in which humans can move, either naturally or as a result of specialized training. Human gait is defined as bipedal forward propulsion of the center of gravity of the human body, in which there are sinuous movements of different segments of the body with little energy spent. Varied gaits are characterized by differences such as limb movement patterns, overall velocity, forces, kinetic and potential energy cycles, and changes in contact with the ground.
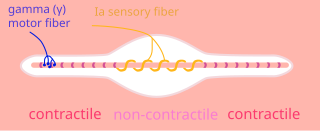
A type Ia sensory fiber, or a primary afferent fiber is a type of afferent nerve fiber. It is the sensory fiber of a stretch receptor called the muscle spindle found in muscles, which constantly monitors the rate at which a muscle stretch changes. The information carried by type Ia fibers contributes to the sense of proprioception.
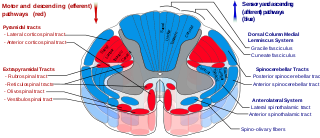
The spinocerebellar tract is a nerve tract originating in the spinal cord and terminating in the same side (ipsilateral) of the cerebellum.
Central pattern generators (CPGs) are self-organizing biological neural circuits that produce rhythmic outputs in the absence of rhythmic input. They are the source of the tightly-coupled patterns of neural activity that drive rhythmic and stereotyped motor behaviors like walking, swimming, breathing, or chewing. The ability to function without input from higher brain areas still requires modulatory inputs, and their outputs are not fixed. Flexibility in response to sensory input is a fundamental quality of CPG-driven behavior. To be classified as a rhythmic generator, a CPG requires:
- "two or more processes that interact such that each process sequentially increases and decreases, and
- that, as a result of this interaction, the system repeatedly returns to its starting condition."

The scratch reflex is a response to activation of sensory neurons whose peripheral terminals are located on the surface of the body. Some sensory neurons can be activated by stimulation with an external object such as a parasite on the body surface. Alternatively, some sensory neurons can respond to a chemical stimulus that produces an itch sensation. During a scratch reflex, a nearby limb reaches toward and rubs against the site on the body surface that has been stimulated. The scratch reflex has been extensively studied to understand the functioning of neural networks in vertebrates. Despite decades of research, key aspects of the scratch reflex are still unknown, such as the neural mechanisms by which the reflex is terminated.

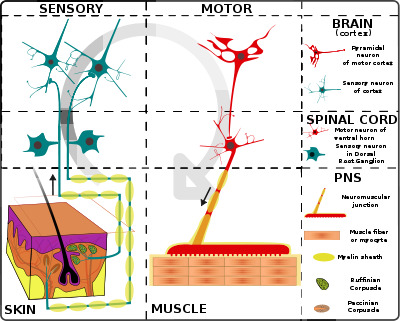
Proprioception, also called kinaesthesia, is the sense of self-movement, force, and body position.

Undulatory locomotion is the type of motion characterized by wave-like movement patterns that act to propel an animal forward. Examples of this type of gait include crawling in snakes, or swimming in the lamprey. Although this is typically the type of gait utilized by limbless animals, some creatures with limbs, such as the salamander, forgo use of their legs in certain environments and exhibit undulatory locomotion. In robotics this movement strategy is studied in order to create novel robotic devices capable of traversing a variety of environments.

Locomotor effects of shoes are the way in which the physical characteristics or components of shoes influence the locomotion neuromechanics of a person. Depending on the characteristics of the shoes, the effects are various, ranging from alteration in balance and posture, muscle activity of different muscles as measured by electromyography (EMG), and the impact force. There are many different types of shoes that exist, such as running, walking, loafers, high heels, sandals, slippers, work boots, dress shoes, and many more. However, a typical shoe will be composed of an insole, midsole, outsole, and heels, if any. In an unshod condition, where one is without any shoes, the locomotor effects are primarily observed in the heel strike patterns and resulting impact forces generated on the ground.
A gait trainer is a wheeled device that assists a person who is unable to walk independently to learn or relearn to walk safely and efficiently as part of gait training. Gait trainers are intended for children or adults with physical disabilities, to provide the opportunity to improve walking ability. A gait trainer offers both unweighting support and postural alignment to enable gait practice. It functions as a support walker and provides more assistance for balance and weight-bearing, than does a traditional rollator walker, or a walker with platform attachments. It also provides opportunities to stand and to bear weight in a safe, supported position.

Central pattern generators are biological neural networks organized to produce any rhythmic output without requiring a rhythmic input. In mammals, locomotor CPGs are organized in the lumbar and cervical segments of the spinal cord, and are used to control rhythmic muscle output in the arms and legs. Certain areas of the brain initiate the descending neural pathways that ultimately control and modulate the CPG signals. In addition to this direct control, there exist different feedback loops that coordinate the limbs for efficient locomotion and allow for the switching of gaits under appropriate circumstances.
When treating a person with a spinal cord injury, repairing the damage created by injury is the ultimate goal. By using a variety of treatments, greater improvements are achieved, and, therefore, treatment should not be limited to one method. Furthermore, increasing activity will increase his/her chances of recovery.

A spinal interneuron, found in the spinal cord, relays signals between (afferent) sensory neurons, and (efferent) motor neurons. Different classes of spinal interneurons are involved in the process of sensory-motor integration. Most interneurons are found in the grey column, a region of grey matter in the spinal cord.
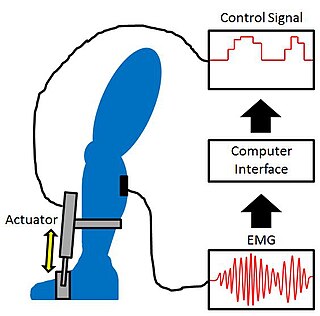
Proportional myoelectric control can be used to activate robotic lower limb exoskeletons. A proportional myoelectric control system utilizes a microcontroller or computer that inputs electromyography (EMG) signals from sensors on the leg muscle(s) and then activates the corresponding joint actuator(s) proportionally to the EMG signal.

Cutaneous, superficial, or skin reflexes, are activated by skin receptors and play a valuable role in locomotion, providing quick responses to unexpected environmental challenges. They have been shown to be important in responses to obstacles or stumbling, in preparing for visually challenging terrain, and for assistance in making adjustments when instability is introduced. In addition to the role in normal locomotion, cutaneous reflexes are being studied for their potential in enhancing rehabilitation therapy (physiotherapy) for people with gait abnormalities.

The Golgi tendon organ (GTO) is a proprioceptor – a type of sensory receptor that senses changes in muscle tension. It lies at the interface between a muscle and its tendon known as the musculotendinous junction also known as the myotendinous junction. It provides the sensory component of the Golgi tendon reflex.
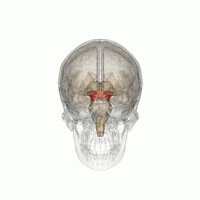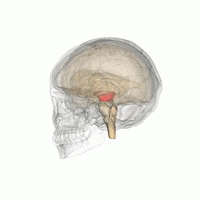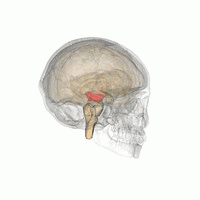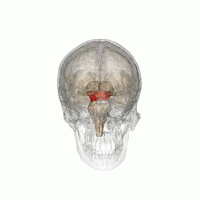
The mesencephalic locomotor region (MLR) is a functionally defined area of the midbrain that is associated with the initiation and control of locomotor movements in vertebrate species.
Postural control refers to the maintenance of body posture in space. The central nervous system interprets sensory input to produce motor output that maintains upright posture. Sensory information used for postural control largely comes from visual, proprioceptive, and vestibular systems. While the ability to regulate posture in vertebrates was previously thought to be a mostly automatic task, controlled by circuits in the spinal cord and brainstem, it is now clear that cortical areas are also involved, updating motor commands based on the state of the body and environment.
Proprioception refers to the sensory information relayed from muscles, tendons, and skin that allows for the perception of the body in space. This feedback allows for more fine control of movement. In the brain, proprioceptive integration occurs in the somatosensory cortex, and motor commands are generated in the motor cortex. In the spinal cord, sensory and motor signals are integrated and modulated by motor neuron pools called central pattern generators (CPGs). At the base level, sensory input is relayed by muscle spindles in the muscle and Golgi tendon organs (GTOs) in tendons, alongside cutaneous sensors in the skin.

Interlimb coordination is the coordination of the left and right limbs. It could be classified into two types of action: bimanual coordination and hands or feet coordination. Such coordination involves various parts of the nervous system and requires a sensory feedback mechanism for the neural control of the limbs. A model can be used to visualize the basic features, the control centre of locomotor movements, and the neural control of interlimb coordination. This coordination mechanism can be altered and adapted for better performance during locomotion in adults and for the development of motor skills in infants. The adaptive feature of interlimb coordination can also be applied to the treatment for CNS damage from stroke and the Parkinson's disease in the future.