
Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes. In viticulture, it is commonly known as "botrytis bunch rot"; in horticulture, it is usually called "grey mould" or "gray mold".

Beauveria bassiana is a fungus that grows naturally in soils throughout the world and acts as a parasite on various arthropod species, causing white muscardine disease; it thus belongs to the group of entomopathogenic fungi. It is used as a biological insecticide to control a number of pests, including termites, thrips, whiteflies, aphids and various beetles. Its use in the control of bedbugs and malaria-transmitting mosquitos is under investigation.
Tolypocladium inflatum is an ascomycete fungus originally isolated from a Norwegian soil sample that, under certain conditions, produces the immunosuppressant drug ciclosporin. In its sexual stage (teleomorph) it is a parasite on scarab beetles. It forms a small, compound ascocarp that arises from the cadaver of its host beetle. In its asexual stage (anamorph) it is a white mold that grows on soil. It is much more commonly found in its asexual stage and this is the stage that was originally given the name Tolypocladium inflatum.
Forma specialis, abbreviated f. sp. without italics, is an informal taxonomic grouping allowed by the International Code of Nomenclature for algae, fungi, and plants, that is applied to a parasite which is adapted to a specific host. This classification may be applied by authors who do not feel that a subspecies or variety name is appropriate, and it is therefore not necessary to specify morphological differences that distinguish this form. The literal meaning of the term is 'special form', but this grouping does not correspond to the more formal botanical use of the taxonomic rank of forma or form.

Alternaria is a genus of Deuteromycetes fungi. All species are known as major plant pathogens. They are also common allergens in humans, growing indoors and causing hay fever or hypersensitivity reactions that sometimes lead to asthma. They are present in the human mycobiome and readily cause opportunistic infections in immunocompromised people such as AIDS patients.
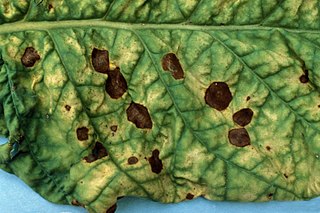
Alternaria alternata is a fungus causing leaf spots, rots, and blights on many plant parts, and other diseases. It is an opportunistic pathogen on over 380 host species of plant.
Alternaria carthami is a necrotrophic plant pathogen of safflower. The fungus is in the order Pleosporales and family Pleosporaceae. It was first isolated in India, has spread globally and can have devastating effects on safflower yield, and resultant oilseed production. A. carthami is known to be seed-borne and appears as irregular brown lesions on safflower leaves and stems.
Alternaria helianthi is a fungal plant pathogen causing a disease in sunflowers known as Alternaria blight of sunflower.

Ulocladium is a genus of fungi. Species of this genus contain both plant pathogens and food spoilage agents. Other species contain enzymes that are biological control agents. Some members of the genus can invade homes and are a sign of moisture because the mold requires water to thrive. They can cause plant diseases or hay fever and more serious infections in immuno-suppressed individuals.
Alternaria penicillata is a species of fungi in the family Pleosporaceae, which causes leaf blight of opium poppy. The fungus is found in Europe, Australia, India, Japan, Nepal, Pakistan, South Africa, Turkey, USA and Zambia.
Wallemia sebi is a xerophilic fungus of the phylum Basidiomycota.
Epicoccum nigrum is a species of fungus in the phylum Ascomycota. A plant pathogen and endophyte, it is a widespread fungus which produces coloured pigments that can be used as antifungal agents against other pathogenic fungi. The fluorescent stain epicocconone is extracted from it.
Alternaria tenuissima is a saprophytic fungus and opportunistic plant pathogen. It is cosmopolitan in distribution, and can colonize a wide range of plant hosts. Colonies of A. tenuissima produce chains on agar growth media. The fungus often forms concentric ring patterns on infected plant leaves. This species produces the allergen Alt a 1, one of the most important outdoor seasonal fungal allergens associated with allergy and asthma provocation. In rare circumstances, this species is also known to infect immunosuppressed humans and animals.

Cladosporium sphaerospermum is a radiotrophic fungus belonging to the genus Cladosporium and was described in 1886 by Albert Julius Otto Penzig from the decaying leaves and branches of Citrus. It is a dematiaceous (darkly-pigmented) fungus characterized by slow growth and largely asexual reproduction. Cladosporium sphaerospermum consists of a complex of poorly morphologically differentiated, "cryptic" species that share many physiological and ecological attributes. In older literature, all of these sibling species were classified as C. sphaerospermum despite their unique nature. Accordingly, there is confusion in older literature reports on the physiological and habitat regularities of C. sphaerospermum in the strict sense. This fungus is most phylogenetically similar to C. fusiforme. According to modern phylogenetic analyses, the previously synonymized species, Cladosporium langeroni, is a distinct species.
Ulocladium chartarum is an ascomycetes mushroom, one of the many in the genus Ulocladium.
Curvularia pallescens is a soil fungus, that commonly grows on crops found in tropical regions. The conidia of the fungus are distinguishable from those of related species due to their lack of curvature. C. pallescens has been reported to cause infection in plants, and in immunocompetent individuals. This species is the anamorph of Cochliobolus pallescens.

Alternaria brassicicola is a fungal necrotrophic plant pathogen that causes black spot disease on a wide range of hosts, particularly in the genus of Brassica, including a number of economically important crops such as cabbage, Chinese cabbage, cauliflower, oilseeds, broccoli and canola. Although mainly known as a significant plant pathogen, it also contributes to various respiratory allergic conditions such as asthma and rhinoconjunctivitis. Despite the presence of mating genes, no sexual reproductive stage has been reported for this fungus. In terms of geography, it is most likely to be found in tropical and sub-tropical regions, but also in places with high rain and humidity such as Poland. It has also been found in Taiwan and Israel. Its main mode of propagation is vegetative. The resulting conidia reside in the soil, air and water. These spores are extremely resilient and can overwinter on crop debris and overwintering herbaceous plants.
Harzia acremonioides is a species of seed-borne fungus that occurs in the soil. It has been categorized in the Ceratostomataceae family and under the genus Harzia. The genus Harzia contained up to three accepted species: H. acremonioides, H. verrucose, and H. velatea in 1974. Within the genus Harzia, H. acremonioides is one of the most common species that can be found in all climate regions around the world.
Oidiodendron cereale is a species of ascomycetes fungi in the order Helotiales. This fungus is found globally in temperate climates where average summer temperatures are below 25 °C, but there have been scattered reports from tropical and subtropical environments. It is predominantly found in soil, but little is known regarding their ecological roles in nature. However, an enzymatic study from Agriculture Canada showed that O. cereale can break down a variety of plant, fungal, and animal based substrates found in soil, which may have beneficial effects for plants. On rare occasions, this fungus is found on human skin and hair. There has been one reported case of O. cereale infection in 1969, causing Neurodermitis Nuchae.

Alternaria leaf spot or Alternaria leaf blight are a group of fungal diseases in plants, that have a variety of hosts. The diseases infects common garden plants, such as cabbage, and are caused by several closely related species of fungi. Some of these fungal species target specific plants, while others have been known to target plant families. One commercially relevant plant genus that can be affected by Alternaria Leaf Spot is Brassica, as the cosmetic issues caused by symptomatic lesions can lead to rejection of crops by distributors and buyers. When certain crops such as cauliflower and broccoli are infected, the heads deteriorate and there is a complete loss of marketability. Secondary soft-rotting organisms can infect stored cabbage that has been affected by Alternaria Leaf Spot by entering through symptomatic lesions. Alternaria Leaf Spot diseases that affect Brassica species are caused by the pathogens Alternaria brassicae and Alternaria brassicicola.









