Related Research Articles

The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the Therapeutic Goods Act 1989, the Therapeutic Goods Regulations 1990, or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods.
The Joint Committee on Vaccination and Immunisation (JCVI) is an independent expert advisory committee that advises United Kingdom health departments on immunisation, making recommendations concerning vaccination schedules and vaccine safety. It has a statutory role in England and Wales, and health departments in Scotland and Northern Ireland may choose to accept its advice.

Nadhim Zahawi is an Iraqi-born British politician who has served as Parliamentary Under-Secretary of State for Business and Industry since 2019 and Parliamentary Under-Secretary of State for COVID-19 Vaccine Deployment since 2020. A member of the Conservative Party, he has been Member of Parliament (MP) for Stratford-on-Avon since 2010.

GAVI, officially Gavi, the Vaccine Alliance is a public–private global health partnership with the goal of increasing access to immunization in poor countries.

The COVID-19 pandemic in the United Kingdom is part of the worldwide pandemic of coronavirus disease 2019 caused by the SARS-CoV-2 virus. The virus reached the UK in late January 2020. As of 30 May 2021, there had been 4.5 million cases confirmed and 128,030 deaths overall among people who had recently tested positive – the world's sixteenth-highest death rate by population and the highest death toll in Europe. There have been 153,371 deaths where the death certificate mentioned COVID by 21 May 2021. There has been some disparity between the outbreak's severity in England, Scotland, Wales and Northern Ireland – health in the UK is devolved, with each of the four having its own publicly funded healthcare system and government.
The COVID-19 pandemic in the Isle of Man is part of the worldwide pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus was confirmed to have reached the British crown dependency of the Isle of Man on 19 March 2020, when a man returning from Spain via Liverpool tested positive. Community transmission was first confirmed on 22 March on the island. By June 2021, there had been 1,598 confirmed cases of COVID-19, of which 1,567 have presumably recovered and 29 have died.

The COVID-19 pandemic reached Northern Ireland on 27 February 2020. The Department of Health reports 2,155 deaths overall among people who had recently tested positive. The Northern Ireland Statistics and Research Agency reports 2,976 where the death certificate mentioned COVID as one possible cause. Northern Ireland has the lowest COVID death rate per population in the United Kingdom. The vast majority of deaths were among those over the age of 75 and almost half were in care homes. Northern Ireland also has a much higher vaccination rate per population than the neighbouring Republic of Ireland.

Her Majesty's Government responded to the COVID-19 pandemic in the United Kingdom in various ways. Because of devolution, following the arrival of COVID-19 on 31 January 2020, the different home nations' administrative responses to the pandemic have been different to one another; the Scottish Government, the Welsh Government, and the Northern Ireland Executive have produced different policies to those that apply in England. The National Health Service is the publicly funded healthcare system of Britain, and has separate branches for each of its four nations.

Operation Warp Speed (OWS) was a public–private partnership initiated by the United States government to facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. The first news report of Operation Warp Speed was on April 29, 2020, and the program was officially announced on May 15, 2020. It was headed by Moncef Slaoui from May 2020 to January 2021 and by David A. Kessler from January to February 2021. At the end of February 2021, Operation Warp Speed was transferred into the responsibilities of the White House COVID-19 Response Team.

The Oxford–AstraZeneca COVID-19 vaccine, codenamed AZD1222, and sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed by Oxford University and AstraZeneca, it is given by intramuscular injection, using as a vector the modified chimpanzee adenovirus ChAdOx1. The efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. Another analysis showed that, for symptomatic COVID-19 infection after the second dose, the vaccine is 66% effective against the Alpha variant, and 60% against the Delta variant.

Nicola Mary Turner is a New Zealand public health advocate who is a professor of General Practice at the University of Auckland and Director of the Immunisation Advisory Centre, the organisation that advises the New Zealand Medical profession and the New Zealand government. She has contributed to advisory committees for the New Zealand Ministry of Health and is a spokesperson for the NZ Child Poverty Action Group. Much of her research and outreach has focused on improving immunisation coverage and closing equity gaps for the national schedule vaccine delivery in New Zealand. Since 2011 Turner has been part of the General Practice team at Newtown Union Health Services (NUHS), Broadway, Wellington.
Dame Catherine Elizabeth Bingham, known as Kate Bingham, is a British venture capitalist. She is a managing partner at a venture capital firm, SV Health Investors.
Helen Petousis-Harris is a New Zealand vaccinologist and associate professor in the Department of General Practice and Primary Health Care at the University of Auckland. She has been involved in research related to vaccination in New Zealand since 1998, with her main areas of focus being vaccine safety and effectiveness. Petousis-Harris has had a variety of lead roles in New Zealand and international organisations that focus on vaccination and is a regular media spokesperson in this field, especially during the COVID-19 pandemic.

The Parliamentary Under-Secretary of State for COVID-19 Vaccine Deployment is a position in the Department of Health and Social Care in the Government of the United Kingdom. It is currently held by Nadhim Zahawi MP who took the office on 28 November 2020. The office was created as a result of the COVID-19 pandemic in the United Kingdom.

The COVID-19 vaccination programme in the United Kingdom is the world's first mass immunisation campaign to protect against SARS-CoV-2 using vaccines developed in response to the COVID-19 pandemic.
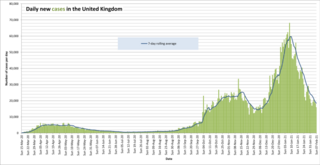
The following is a timeline of the COVID-19 pandemic in the United Kingdom during 2021. There are significant differences in the legislation and the reporting between the countries of the UK: England, Scotland, Northern Ireland, and Wales. The numbers of cases and deaths are reported on a government Web site updated daily during the pandemic. The UK-wide COVID Symptom Study based on surveys of four million participants, endorsed by authorities in Scotland and Wales, run by health science company ZOE, and analysed by King's College London researchers, publishes daily estimates of the number of new and total current COVID-19 infections in UK regions, without restriction to only laboratory-confirmed cases.

The general COVID-19 vaccination in Australia programme began on 22 February 2021, and will continue with the goal of vaccinating all willing Australians before 2022. Front-line workers and aged care staff and residents will be the first Australians to be inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) has approved two vaccines in Australia: the Pfizer–BioNTech vaccine on 25 January, and the Oxford–AstraZeneca vaccine on 16 February. As of 15 June 2021, Australia has administered 5,931,245 vaccine doses across the country.

COVID-19 vaccination in the Republic of Ireland began on 29 December 2020, in response to the ongoing pandemic in the Republic of Ireland. As of 11 May 2021, 1,408,105 people had received the first dose of a vaccine and 514,808 had received their second dose, bringing the total of vaccines administered to 1,922,913.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
References
- 1 2 "Kate Bingham appointed chair of UK Vaccine Taskforce". GOV.UK OGL v3.0. 16 May 2020.
- ↑ "Government launches Vaccine Taskforce to combat coronavirus". gov.uk. 17 April 2020. Retrieved 6 October 2020.
- ↑ Vaccine Taskforce Aims (PDF) (Report). www.gov.uk. 6 April 2020. p. 1. Retrieved 6 October 2020.
Currently there is work going on across government but it is not sufficiently coordinated. The taskforce will bring together government, industry, academics, funding agencies, regulators, logistics and finance to make rapid decisions to put the UK in a position to accelerate vaccine development and vaccinate the right proportion of the population as soon as possible after a vaccine is available.
- ↑ Boseley, Sarah (1 July 2020). "Oxford offers best hope for Covid-19 vaccine this year, MPs told". Guardian News & Media Limited.
- ↑ Boland, Hannah (12 September 2020). "Oxford's Sir John Bell: 'We're not going to beat the second wave'". Telegraph Media Group Limited.
- ↑ "Coronavirus vaccine will be only 50pc effective, warns head of UK taskforce". The Telegraph. London. 14 October 2020. Retrieved 14 October 2020.
- ↑ Sandhu, Rajdeep (17 October 2020). "Covid-19: Most vulnerable 'could get vaccine by Christmas'". BBC Scotland. Retrieved 18 October 2020.
- ↑ Hayes, Andy (18 October 2020). "Coronavirus: 'More than one vaccine' will be available early in 2021, SAGE scientist says". Sky News. Retrieved 18 October 2020.
- ↑ "Expert partnership to explore and establish Human Challenge studies of COVID-19 in the UK" (Press release). London. www.gov.uk. 20 October 2020. Retrieved 24 October 2020.
- ↑ "Nadhim Zahawi MP". GOV.UK. Retrieved 2 March 2021.
- ↑ "Update on the Vaccine Taskforce: 1 March 2021". GOV.UK. 1 March 2021. Retrieved 2 March 2021.
- ↑ "Sir Richard Sykes appointed chair of Vaccine Taskforce". GOV.UK. 14 June 2021. Retrieved 15 June 2021.
- ↑ Pogrund, Gabriel (7 November 2020). "Vaccine tsar Kate Bingham runs up £670,000 PR bill". The Times . ISSN 0140-0460 . Retrieved 2 May 2021.
- ↑ "VTF objectives and membership of the steering group". GOV.UK. Department for Business, Energy & Industrial Strategy. 24 November 2020. Retrieved 15 June 2021.
- ↑ Cookson, Clive (20 October 2020). "Volunteers to be infected with coronavirus in world's first 'human challenge' trials". The Financial Times. London. Retrieved 24 October 2020.
- ↑ "Expert partnership to explore and establish Human Challenge studies of COVID-19 in the UK" (Press release). London. www.gov.uk. 20 October 2020. Retrieved 24 October 2020.
Innovation Minister Lord Bethell said: "This investment into new facilities at PHE Porton Down will enable its dedicated and expert scientists to accelerate the pace and scale of specialised testing to support the critical work of the Vaccine Taskforce."
- ↑ "UK Vaccines Taskforce has Selected Oxford Immunotec as the Sole Supplier of T cell Testing for SARS-CoV-2 Specific Responses in New COVID Vaccine Trials" (Press release). Oxford. Globe Newswire. 22 October 2020. Retrieved 22 October 2020.
- ↑ Bingham, Kate (27 October 2020). "The UK Government's Vaccine Taskforce: strategy for protecting the UK and the world". The Lancet. 397 (10268): 68–70. doi: 10.1016/S0140-6736(20)32175-9 . PMC 7833709 . PMID 33125932. S2CID 225080217.
- ↑ "Coronavirus vaccine taskforce chair says first COVID vaccines 'likely to be imperfect' and 'might not prevent infection'". Sky News. 28 October 2020. Retrieved 28 October 2020.