Related Research Articles
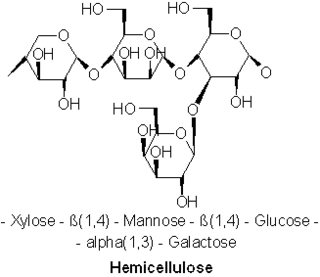
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Dictyoglomus is a genus of bacterium, given its own Phylum, called the Dictyoglomi. This organism is extremely thermophilic, meaning it thrives at extremely high temperatures. It is chemoorganotrophic, meaning it derives energy by metabolizing organic molecules. This organism is of interest because it elaborates an enzyme, xylanase, which digests xylan, a heteropolymer of the pentose sugar xylose. By pretreating wood pulp with this enzyme, paper manufacturers can achieve comparable levels of whiteness with much less chlorine bleach.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
In enzymology, a vanillin synthase is an enzyme that catalyzes the chemical reaction
The enzyme ectoine synthase (EC ) catalyzes the chemical reaction
The enzyme hyaluronate lyase catalyzes the chemical reaction
The enzyme mannuronate-specific alginate lyase catalyzes the degradation of alginate into various monosaccharide and polysaccharide products:
In enzymology, a trans-feruloyl-CoA hydratase (EC 4.2.1.101) is an enzyme that catalyzes the chemical reaction
In enzymology, a capsular-polysaccharide-transporting ATPase (EC 7.6.2.2) is an enzyme that catalyzes the chemical reaction
The enzyme feruloyl esterase (EC 3.1.1.73) catalyzes the reaction
In enzymology, a diaminobutyrate acetyltransferase (EC 2.3.1.178) is an enzyme that catalyzes the chemical reaction
In enzymology, a diaminobutyrate-2-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction

Paenibacillus vortex is a species of pattern-forming bacteria, first discovered in the early 1990s by Eshel Ben-Jacob's group at Tel Aviv University. It is a social microorganism that forms colonies with complex and dynamic architectures. P. vortex is mainly found in heterogeneous and complex environments, such as the rhizosphere, the soil region directly influenced by plant roots.

Paenibacillus dendritiformis is a species of pattern-forming bacteria, first discovered in the early 90s by Eshel Ben-Jacob's group. It is a social microorganism that forms colonies with complex and dynamic architectures. The genus Paenibacillus comprises facultative anaerobic, endospore-forming bacteria originally included within the genus Bacillus and then reclassified as a separate genus in 1993. Bacteria belonging to this genus have been detected in a variety of environments such as: soil, water, rhizosphere, vegetable matter, forage and insect larvae.
In molecular biology, glycoside hydrolase family 88 is a family of glycoside hydrolases.

Glucan 1,4-alpha-maltohydrolase is an enzyme with systematic name 4-alpha-D-glucan alpha-maltohydrolase. This enzyme catalyses the following chemical reaction
Gellan tetrasaccharide unsaturated glucuronyl hydrolase (EC 3.2.1.179, UGL, unsaturated glucuronyl hydrolase) is an enzyme with systematic name beta-D-4-deoxy-Delta4-GlcAp-(1->4)-beta-D-Glcp-(1->4)-alpha-L-Rhap-(1->3)-beta-D-Glcp beta-D-4-deoxy-Delta4-GlcAp hydrolase. This enzyme catalyses the following chemical reaction
Rhamnogalacturonan exolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate exolyase. This enzyme catalyses the following chemical reaction
The enzyme Rhamnogalacturonan endolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate endolyase. catalyses the following process:
Gellan lyase is an enzyme with systematic name gellan β-D-glucopyranosyl-(1→4)-D-glucopyranosyluronate lyase. This enzyme catalyses the following process:
References
- ↑ Sutherland IW (1987). "Xanthan lyases--novel enzymes found in various bacterial species". J. Gen. Microbiol. 133 (11): 3129–34. doi: 10.1099/00221287-133-11-3129 . PMID 3446747.
- 1 2 Hashimoto W, Miki H, Tsuchiya N, Nankai H, Murata K (1 October 1998). "Xanthan lyase of Bacillus sp. strain GL1 liberates pyruvylated mannose from xanthan side chains". Appl. Environ. Microbiol. 64 (10): 3765–8. doi:10.1128/AEM.64.10.3765-3768.1998. PMC 106543 . PMID 9758797.
- 1 2 Ruijssenaars HJ, de Bont JA, Hartmans S (1 June 1999). "A pyruvated mannose-specific xanthan lyase involved in xanthan degradation by Paenibacillus alginolyticus XL-1". Appl. Environ. Microbiol. 65 (6): 2446–52. doi:10.1128/AEM.65.6.2446-2452.1999. PMC 91360 . PMID 10347025.
- ↑ Ostrowski, Matthew P.; La Rosa, Sabina Leanti; Kunath, Benoit J.; Robertson, Andrew; Pereira, Gabriel; Hagen, Live H.; Varghese, Neha J.; Qiu, Ling; Yao, Tianming; Flint, Gabrielle; Li, James; McDonald, Sean P.; Buttner, Duna; Pudlo, Nicholas A.; Schnizlein, Matthew K.; Young, Vincent B.; Brumer, Harry; Schmidt, Thomas M.; Terrapon, Nicolas; Lombard, Vincent; Henrissat, Bernard; Hamaker, Bruce; Eloe-Fadrosh, Emiley A.; Tripathi, Ashootosh; Pope, Phillip B.; Martens, Eric C. (April 2022). "Mechanistic insights into consumption of the food additive xanthan gum by the human gut microbiota". Nature Microbiology. 7 (4): 556–569. doi:10.1038/s41564-022-01093-0. PMID 35365790. S2CID 247866305.
- ↑ Hashimoto W, Nankai H, Mikami B, Murata K (2003). "Crystal structure of Bacillus sp. GL1 xanthan lyase, which acts on the side chains of xanthan". J. Biol. Chem. 278 (9): 7663–73. doi: 10.1074/jbc.M208100200 . PMID 12475987.
- Sutherland IW (1987). "Xanthan lyases--novel enzymes found in various bacterial species". J. Gen. Microbiol. 133 (11): 3129–34. doi: 10.1099/00221287-133-11-3129 . PMID 3446747.