Related Research Articles

Tumor necrosis factor is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.

Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the GCG gene.

The tumor necrosis factor receptor superfamily (TNFRSF) is a protein superfamily of cytokine receptors characterized by the ability to bind tumor necrosis factors (TNFs) via an extracellular cysteine-rich domain. With the exception of nerve growth factor (NGF), all TNFs are homologous to the archetypal TNF-alpha. In their active form, the majority of TNF receptors form trimeric complexes in the plasma membrane. Accordingly, most TNF receptors contain transmembrane domains (TMDs), although some can be cleaved into soluble forms, and some lack a TMD entirely. In addition, most TNF receptors require specific adaptor protein such as TRADD, TRAF, RIP and FADD for downstream signalling. TNF receptors are primarily involved in apoptosis and inflammation, but they can also take part in other signal transduction pathways, such as proliferation, survival, and differentiation. TNF receptors are expressed in a wide variety of tissues in mammals, especially in leukocytes.

Sheddases are membrane-bound enzymes that cleave extracellular portions of transmembrane proteins, releasing the soluble ectodomains from the cell surface. Many sheddases are members of the ADAM or aspartic protease (BACE) protein families.

A disintegrin and metalloprotease 17 (ADAM17), also called TACE, is a 70-kDa enzyme that belongs to the ADAM protein family of disintegrins and metalloproteases.
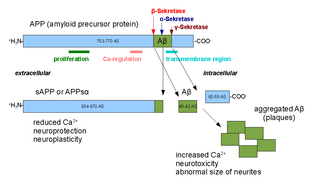
Alpha secretases are a family of proteolytic enzymes that cleave amyloid precursor protein (APP) in its transmembrane region. Specifically, alpha secretases cleave within the fragment that gives rise to the Alzheimer's disease-associated peptide amyloid beta when APP is instead processed by beta secretase and gamma secretase. The alpha-secretase pathway is the predominant APP processing pathway. Thus, alpha-secretase cleavage precludes amyloid beta formation and is considered to be part of the non-amyloidogenic pathway in APP processing. Alpha secretases are members of the ADAM family, which are expressed on the surfaces of cells and anchored in the cell membrane. Several such proteins, notably ADAM10, have been identified as possessing alpha-secretase activity. Upon cleavage by alpha secretases, APP releases its extracellular domain - a fragment known as APPsα - into the extracellular environment in a process known as ectodomain shedding.

Syndecan 1 is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

Matrilysin also known as matrix metalloproteinase-7 (MMP-7), pump-1 protease (PUMP-1), or uterine metalloproteinase is an enzyme in humans that is encoded by the MMP7 gene. The enzyme has also been known as matrin, putative metalloproteinase-1, matrix metalloproteinase pump 1, PUMP-1 proteinase, PUMP, metalloproteinase pump-1, putative metalloproteinase, MMP). Human MMP-7 has a molecular weight around 30 kDa.
Membrane-bound transcription factor site-2 protease, also known as S2P endopeptidase or site-2 protease (S2P), is an enzyme encoded by the MBTPS2 gene which liberates the N-terminal fragment of sterol regulatory element-binding protein (SREBP) transcription factors from membranes. S2P cleaves the transmembrane domain of SREPB, making it a member of the class of intramembrane proteases.
Kexin is a prohormone-processing protease, specifically a yeast serine peptidase, found in the budding yeast. It catalyzes the cleavage of -Lys-Arg- and -Arg-Arg- bonds to process yeast alpha-factor pheromone and killer toxin precursors. The human homolog is PCSK4. It is a family of subtilisin-like peptidases. Even though there are a few prokaryote kexin-like peptidases, all kexins are eukaryotes. The enzyme is encoded by the yeast gene KEX2, and usually referred to in the scientific community as Kex2p. It shares structural similarities with the bacterial protease subtilisin. The first mammalian homologue of this protein to be identified was furin. In the mammal, kexin-like peptidases function in creating and regulating many differing proproteins.

A Disintegrin and metalloproteinase domain-containing protein 10, also known as ADAM10 or CDw156 or CD156c is a protein that in humans is encoded by the ADAM10 gene.

Fibroblast activation protein alpha (FAP-alpha) also known as prolyl endopeptidase FAP is an enzyme that in humans is encoded by the FAP gene.

ADAM19 , is a human gene.

Matrix metalloproteinase-17 (MMP-17) also known as membrane-type matrix metalloproteinase 4 is an enzyme that in humans is encoded by the MMP17 gene.

Death receptor 6 (DR6), also known as tumor necrosis factor receptor superfamily member 21 (TNFRSF21), is a cell surface receptor of the tumor necrosis factor receptor superfamily which activates the JNK and NF-κB pathways. It is mostly expressed in the thymus, spleen and white blood cells. The Gene for DR6 is 78,450 bases long and is found on the 6th chromosome. This is transcribed into a 655 amino acid chain weighing 71.8 kDa. Post transcriptional modifications of this protein include glycosylation on the asparagines at the 82, 141, 252, 257, 278, and 289 amino acid locations.

Tumor necrosis factor receptor 2 (TNFR2), also known as tumor necrosis factor receptor superfamily member 1B (TNFRSF1B) and CD120b, is one of two membrane receptors that binds tumor necrosis factor-alpha (TNFα). Like its counterpart, tumor necrosis factor receptor 1 (TNFR1), the extracellular region of TNFR2 consists of four cysteine-rich domains which allow for binding to TNFα. TNFR1 and TNFR2 possess different functions when bound to TNFα due to differences in their intracellular structures, such as TNFR2 lacking a death domain (DD).
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
Infectious pancreatic necrosis birnavirus Vp4 peptidase (EC 3.4.21.115, infectious pancreatic necrosis virus protease, IPNV Vp4 protease, IPNV Vp4 peptidase, NS protease, NS-associated protease, Vp4 protease) is an enzyme. This enzyme catalyses the following chemical reaction
ADAMTS-4 endopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Black RA (January 2002). "Tumor necrosis factor-alpha converting enzyme". The International Journal of Biochemistry & Cell Biology. 34 (1): 1–5. doi:10.1016/S1357-2725(01)00097-8. PMID 11733179.