Related Research Articles

Crotalus is a genus of venomous pit vipers in the family Viperidae, commonly known as rattlesnakes or rattlers. The genus is found only in the Americas from southern Canada to northern Argentina. The generic name Crotalus is derived from the Greek word κρόταλονkrótalοn, which means "rattle" or "castanet", and refers to the rattle on the end of the tail, which makes this group so distinctive. As of July 2023, 44 to 53 species are recognized as being valid.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is injected by unique fangs during a bite, whereas some species are also able to spit venom.

Crotalus scutulatus is a highly venomous pit viper species found in the deserts of the southwestern United States and central Mexico. It is perhaps best known for its potent neurotoxic-hemotoxic venom, which is considered the world's most potent rattlesnake venom.
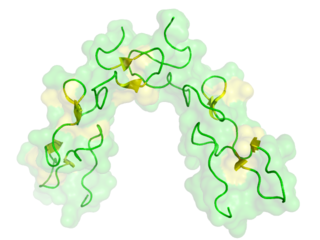
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.

The eastern diamondback rattlesnake is a species of pit viper in the family Viperidae. The species is endemic to the Southeastern United States. It is one of the heaviest venomous snakes in the Americas and the largest rattlesnake. No subspecies are recognized.

Myotoxins are small, basic peptides found in snake venoms and lizard venoms. This involves a non-enzymatic mechanism that leads to severe muscle necrosis. These peptides act very quickly, causing instantaneous paralysis to prevent prey from escaping and eventually death due to diaphragmatic paralysis.

Crotalus ruber is a venomous pit viper species found in southwestern California in the United States and Baja California in Mexico. Three subspecies are currently recognized, including the nominate subspecies described here.

The western diamondback rattlesnake or Texas diamond-back is a rattlesnake species and member of the viper family, found in the southwestern United States and Mexico. Like all other rattlesnakes and all other vipers, it is venomous. It is likely responsible for the majority of snakebite fatalities in northern Mexico and the greatest number of snakebites in the U.S. No subspecies are currently recognized.
Adamalysin is an enzyme. This enzyme catalyses the following chemical reaction

Crotamine is a toxin present in the venom of the South American rattlesnake. It is a 42-residue long protein containing 11 basic residues and 6 cysteines. It has also been isolated from the venom of North American prairie rattlesnake, Crotalus viridis viridis. It was first isolated and purified by Brazilian scientist José Moura Gonçalves, and later intensively studied by his group of collaborators at the Medical School of Ribeirão Preto of the University of São Paulo.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
Atrolysin B is an enzyme. This enzyme catalyses the following chemical reaction
Atroxase is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin E is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin F is an enzyme. This enzyme catalyses the following chemical reaction
Horrilysin is an enzyme. This enzyme catalyses the following chemical reaction
Ruberlysin is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin II is an enzyme. This enzyme catalyses the following chemical reaction
Mucrolysin is an enzyme. This enzyme catalyses the following chemical reaction

Venom in snakes and some lizards is a form of saliva that has been modified into venom over its evolutionary history. In snakes, venom has evolved to kill or subdue prey, as well as to perform other diet-related functions. While snakes occasionally use their venom in self defense, this is not believed to have had a strong effect on venom evolution. The evolution of venom is thought to be responsible for the enormous expansion of snakes across the globe.
References
- ↑ Bjarnason JB, Tu AT (August 1978). "Hemorrhagic toxins from Western diamondback rattlesnake (Crotalus atrox) venom: isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e". Biochemistry. 17 (16): 3395–404. doi:10.1021/bi00609a033. PMID 210790.
- ↑ Fox JW, Campbell R, Beggerly L, Bjarnason JB (April 1986). "Substrate specificities and inhibition of two hemorrhagic zinc proteases Ht-c and Ht-d from Crotalus atrox venom". European Journal of Biochemistry. 156 (1): 65–72. doi: 10.1111/j.1432-1033.1986.tb09549.x . PMID 3514216.
- ↑ Bjarnason JB, Fox JW (February 1987). "Characterization of two hemorrhagic zinc proteinases, toxin c and toxin d, from western diamondback rattlesnake (Crotalus atrox) venom". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 911 (3): 356–63. doi:10.1016/0167-4838(87)90077-x. PMID 3101740.
- ↑ Mori N, Nikai T, Sugihara H, Tu AT (February 1987). "Biochemical characterization of hemorrhagic toxins with fibrinogenase activity isolated from Crotalus ruber ruber venom". Archives of Biochemistry and Biophysics. 253 (1): 108–21. doi:10.1016/0003-9861(87)90643-6. PMID 2949699.
- ↑ Shannon JD, Baramova EN, Bjarnason JB, Fox JW (July 1989). "Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin". The Journal of Biological Chemistry. 264 (20): 11575–83. PMID 2745407.
- ↑ Takeya H, Onikura A, Nikai T, Sugihara H, Iwanaga S (November 1990). "Primary structure of a hemorrhagic metalloproteinase, HT-2, isolated from the venom of Crotalus ruber ruber". Journal of Biochemistry. 108 (5): 711–9. doi:10.1093/oxfordjournals.jbchem.a123270. PMID 2081731.