Related Research Articles
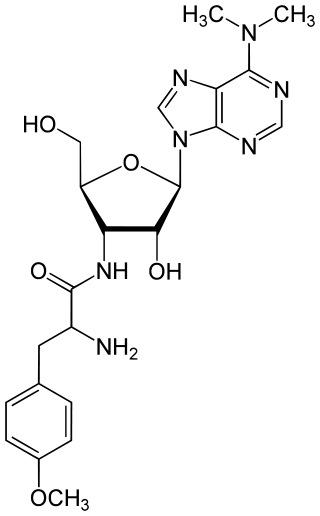
Puromycin is an antibiotic protein synthesis inhibitor which causes premature chain termination during translation.
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal, an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide.
Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 is a protein that, in humans, is encoded by the DPP4 gene. DPP4 is related to FAP, DPP8, and DPP9. The enzyme was discovered in 1966 by Hopsu-Havu and Glenner, and as a result of various studies on chemism, was called dipeptidyl peptidase IV [DP IV].
Dipeptidyl peptidase is a type of enzyme classified under EC 3.4.14.
Glutamyl aminopeptidase (EC 3.4.11.7, aminopeptidase A, aspartate aminopeptidase, angiotensinase A, glutamyl peptidase, Ca2+-activated glutamate aminopeptidase, membrane aminopeptidase II, antigen BP-1/6C3 of mouse B lymphocytes, L-aspartate aminopeptidase, angiotensinase A2) is an enzyme encoded by the ENPEP gene. Glutamyl aminopeptidase has also recently been designated CD249 (cluster of differentiation 249).

Dipeptidyl-peptidase 3 is an enzyme that in humans is encoded by the DPP3 gene.

Dipeptidyl-peptidase 2 is an enzyme that in humans is encoded by the DPP7 gene.

Dipeptidyl peptidase 8 is an enzyme that in humans is encoded by the DPP8 gene.

Dipeptidyl peptidase 9 is an enzyme that in humans is encoded by the DPP9 gene.

Dipeptidyl peptidase I is an enzyme. This enzyme catalyses the following chemical reaction
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:
Dipeptidyl aminopeptidase III may refer to:
Dipeptidyl-peptidase III is an enzyme. This enzyme catalyses the following chemical reaction

Tynorphin is a synthetic opioid peptide which is a potent and competitive inhibitor of the enkephalinase class of enzymes which break down the endogenous enkephalin peptides. It specifically inactivates dipeptidyl aminopeptidase III (DPP3) with very high efficacy, but also inhibits neutral endopeptidase (NEP), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE) to a lesser extent. It has a pentapeptide structure with the amino acid sequence Val-Val-Tyr-Pro-Trp (VVYPW).
Tripeptide aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction:
Bacterial leucyl aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Dipeptidyl-dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Xaa-Pro dipeptidyl-peptidase (EC 3.4.14.11, X-prolyl dipeptidyl aminopeptidase, PepX, X-prolyl dipeptidyl peptidase is an enzyme. It catalyses the following chemical reaction
Pyroglutamyl-peptidase I (EC 3.4.19.3, also known as Pyrrolidonyl peptidase, is an enzyme found in bacteria, plants and animals.
An endopeptidase inhibitor is a drug that inhibits one or more endopeptidase enzymes. Endopeptidases are one of two types of proteases, the other being exopeptidases. Endopeptidases cleave peptide bonds of non-terminal amino acids, whereas exopeptidases break terminal bonds, resulting in the release of a single amino acid or dipeptide from the peptide chain.
References
- ↑ McDonald JK, Leibach FH, Grindeland RE, Ellis S (August 1968). "Purification of dipeptidyl aminopeptidase II (dipeptidyl arylamidase II) of the anterior pituitary gland. Peptidase and dipeptide esterase activities". The Journal of Biological Chemistry. 243 (15): 4143–50. doi: 10.1016/S0021-9258(18)93291-6 . PMID 4969969.
- ↑ McDonald JK, Schwabe C (1977). "Intracellular exopeptidases". In Barrett AJ (ed.). Proteinases in Mammalian Cells and Tissues. Amsterdam: North-Holland Publishing Co. pp. 311–391.