
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
Micellar electrokinetic chromatography (MEKC) is a chromatography technique used in analytical chemistry. It is a modification of capillary electrophoresis (CE), extending its functionality to neutral analytes, where the samples are separated by differential partitioning between micelles and a surrounding aqueous buffer solution.
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH.

Ion chromatography separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography - MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC-MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC-MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC-MS has also begun to be used in clinical applications.
Electrochromatography is a chemical separation technique in analytical chemistry, biochemistry and molecular biology used to resolve and separate mostly large biomolecules such as proteins. It is a combination of size exclusion chromatography and gel electrophoresis. These separation mechanisms operate essentially in superposition along the length of a gel filtration column to which an axial electric field gradient has been added. The molecules are separated by size due to the gel filtration mechanism and by electrophoretic mobility due to the gel electrophoresis mechanism. Additionally there are secondary chromatographic solute retention mechanisms.

Thermospray is a soft ionization source by which a solvent flow of liquid sample passes through a very thin heated column to become a spray of fine liquid droplets. As a form of atmospheric pressure ionization in mass spectrometry these droplets are then ionized via a low-current discharge electrode to create a solvent ion plasma. A repeller then directs these charged particles through the skimmer and acceleration region to introduce the aerosolized sample to a mass spectrometer. It is particularly useful in liquid chromatography-mass spectrometry (LC-MS).

Laser spray ionization refers to one of several methods for creating ions using a laser interacting with a spray of neutral particles or ablating material to create a plume of charged particles. The ions thus formed can be separated by m/z with mass spectrometry. Laser spray is one of several ion sources that can be coupled with liquid chromatography-mass spectrometry for the detection of larger molecules.

Two-dimensional chromatography is a type of chromatographic technique in which the injected sample is separated by passing through two different separation stages. Two different chromatographic columns are connected in sequence, and the effluent from the first system is transferred onto the second column. Typically the second column has a different separation mechanism, so that bands that are poorly resolved from the first column may be completely separated in the second column. Alternately, the two columns might run at different temperatures. During the second stage of separation the rate at which the separation occurs must be faster than the first stage, since there is still only a single detector. The plane surface is amenable to sequential development in two directions using two different solvents.

Capillary electrophoresis–mass spectrometry (CE-MS) is an analytical chemistry technique formed by the combination of the liquid separation process of capillary electrophoresis with mass spectrometry. CE-MS combines advantages of both CE and MS to provide high separation efficiency and molecular mass information in a single analysis. It has high resolving power and sensitivity, requires minimal volume and can analyze at high speed. Ions are typically formed by electrospray ionization, but they can also be formed by matrix-assisted laser desorption/ionization or other ionization techniques. It has applications in basic research in proteomics and quantitative analysis of biomolecules as well as in clinical medicine. Since its introduction in 1987, new developments and applications have made CE-MS a powerful separation and identification technique. Use of CE-MS has increased for protein and peptides analysis and other biomolecules. However, the development of online CE-MS is not without challenges. Understanding of CE, the interface setup, ionization technique and mass detection system is important to tackle problems while coupling capillary electrophoresis to mass spectrometry.
Bioanalysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics and biotics in biological systems.
The history of electrophoresis for molecular separation and chemical analysis began with the work of Arne Tiselius in 1931, while new separation processes and chemical analysis techniques based on electrophoresis continue to be developed in the 21st century. Tiselius, with support from the Rockefeller Foundation, developed the "Tiselius apparatus" for moving boundary electrophoresis, which was described in 1937 in the well-known paper "A New Apparatus for Electrophoretic Analysis of Colloidal Mixtures". The method spread slowly until the advent of effective zone electrophoresis methods in the 1940s and 1950s, which used filter paper or gels as supporting media. By the 1960s, increasingly sophisticated gel electrophoresis methods made it possible to separate biological molecules based on minute physical and chemical differences, helping to drive the rise of molecular biology. Gel electrophoresis and related techniques became the basis for a wide range of biochemical methods, such as protein fingerprinting, Southern blot, other blotting procedures, DNA sequencing, and many more.
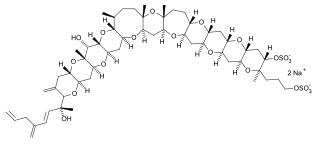
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substance in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.

Capillary electrochromatography (CEC) is a chromatographic technique in which the mobile phase is driven through the chromatographic bed by electroosmosis. Capillary electrochromatography is a combination of two analytical techniques, high-performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis aims to separate analytes on the basis of their mass-to-charge ratio by passing a high voltage across ends of a capillary tube, which is filled with the analyte. High-performance liquid chromatography separates analytes by passing them, under high pressure, through a column filled with stationary phase. The interactions between the analytes and the stationary phase and mobile phase lead to the separation of the analytes. In capillary electrochromatography capillaries, packed with HPLC stationary phase, are subjected to a high voltage. Separation is achieved by electrophoretic migration of solutes and differential partitioning.
Electro membrane extraction, or EME, is a miniaturized liquid-liquid extraction technique developed for sample preparation of aqueous samples prior to analysis by chromatography, electrophoresis, mass spectrometry, and related techniques in analytical chemistry. EME involves the use of a small supported liquid membrane (SLM) sustained in the wall of a porous hollow fiber, and application of an electrical field across the SLM.

James Wallace Jorgenson is an American academic who previously held the position of William Rand Kenan Jr. Distinguished Professor of Chemistry at UNC-Chapel Hill. He is best known for his work developing capillary zone electrophoresis, and is a member of the American Academy of Arts and Sciences.















