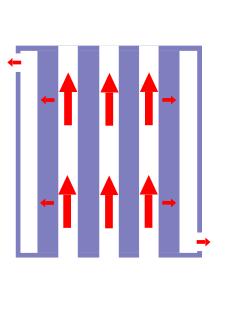
Electrofiltration is a method that combines membrane filtration and electrophoresis in a dead-end process.
Membrane technology covers all engineering approaches for the transport of substances between two fractions with the help of permeable membranes. In general, mechanical separation processes for separating gaseous or liquid streams use membrane technology.

Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. Electrophoresis of positively charged particles (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles (anions) is sometimes called anaphoresis.
Contents
Electrofiltration is regarded as an appropriate technique for concentration and fractionation of biopolymers. The film formation on the filter membrane which hinders filtration can be minimized or completely avoided by the application of electric field, improving filtration’s performance and increasing selectivity in case of fractionation. This approach reduces significantly the expenses for downstream processing in bioprocesses.

Biopolymers are polymers produced by living organisms; in other words, they are polymeric biomolecules. Biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers, classified according to the monomeric units used and the structure of the biopolymer formed: polynucleotides, which are long polymers composed of 13 or more nucleotide monomers; polypeptides, which are short polymers of amino acids; and polysaccharides, which are often linear bonded polymeric carbohydrate structures. Other examples of biopolymers include rubber, suberin, melanin and lignin.
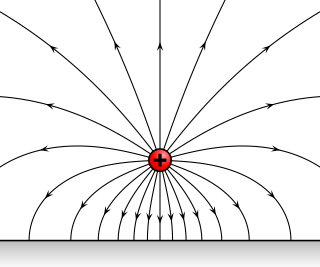
An electric field surrounds an electric charge, and exerts force on other charges in the field, attracting or repelling them. Electric field is sometimes abbreviated as E-field. The electric field is defined mathematically as a vector field that associates to each point in space the force per unit of charge exerted on an infinitesimal positive test charge at rest at that point. The SI unit for electric field strength is volt per meter (V/m). Newtons per coulomb (N/C) is also used as a unit of electric field strength. Electric fields are created by electric charges, or by time-varying magnetic fields. Electric fields are important in many areas of physics, and are exploited practically in electrical technology. On an atomic scale, the electric field is responsible for the attractive force between the atomic nucleus and electrons that holds atoms together, and the forces between atoms that cause chemical bonding. Electric fields and magnetic fields are both manifestations of the electromagnetic force, one of the four fundamental forces of nature.
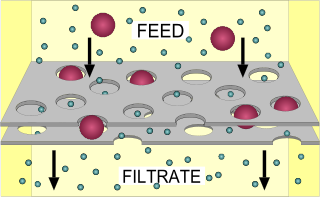
Filtration is any of various mechanical, physical or biological operations that separates solids from fluids by adding a medium through which only the fluid can pass. The fluid that passes through is called the filtrate. In physical filters oversize solids in the fluid are retained and in biological filters particulates are trapped and ingested and metabolites are retained and removed. However, the separation is not complete; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms. For example, in animals, renal filtration removes waste from the blood, and in water treatment and sewage treatment, undesirable constituents are removed by people into a animal film grown on or in the filter medium, as in slow sand filtration.