Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.

Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate.

Settling is the process by which particulates move towards the bottom of a liquid and form a sediment. Particles that experience a force, either due to gravity or due to centrifugal motion will tend to move in a uniform manner in the direction exerted by that force. For gravity settling, this means that the particles will tend to fall to the bottom of the vessel, forming sludge or slurry at the vessel base. Settling is an important operation in many applications, such as mining, wastewater and drinking water treatment, biological science, space propellant reignition, and scooping.

Wastewater treatment is a process which removes and eliminates contaminants from wastewater. It thus converts it into an effluent that can be returned to the water cycle. Once back in the water cycle, the effluent creates an acceptable impact on the environment. It is also possible to reuse it. This process is called water reclamation. The treatment process takes place in a wastewater treatment plant. There are several kinds of wastewater which are treated at the appropriate type of wastewater treatment plant. For domestic wastewater the treatment plant is called a Sewage Treatment. Municipal wastewater or sewage are other names for domestic wastewater. For industrial wastewater, treatment takes place in a separate Industrial wastewater treatment, or in a sewage treatment plant. In the latter case it usually follows pre-treatment. Further types of wastewater treatment plants include Agricultural wastewater treatment and leachate treatment plants.

A settling basin, settling pond or decant pond is an earthen or concrete structure using sedimentation to remove settleable matter and turbidity from wastewater. The basins are used to control water pollution in diverse industries such as agriculture, aquaculture, and mining. Turbidity is an optical property of water caused by scattering of light by material suspended in that water. Although turbidity often varies directly with weight or volumetric measurements of settleable matter, correlation is complicated by variations in size, shape, refractive index, and specific gravity of suspended matter. Settling ponds may be ineffective at reducing turbidity caused by small particles with specific gravity low enough to be suspended by Brownian motion.

Sedimentation is the deposition of sediments. It takes place when particles in suspension settle out of the fluid in which they are entrained and come to rest against a barrier. This is due to their motion through the fluid in response to the forces acting on them: these forces can be due to gravity, centrifugal acceleration, or electromagnetism. Settling is the falling of suspended particles through the liquid, whereas sedimentation is the final result of the settling process.

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.

The activated sludgeprocess is a type of biological wastewater treatment process for treating sewage or industrial wastewaters using aeration and a biological floc composed of bacteria and protozoa. It is one of several biological wastewater treatment alternatives in secondary treatment, which deals with the removal of biodegradable organic matter and suspended solids. It uses air and microorganisms to biologically oxidize organic pollutants, producing a waste sludge containing the oxidized material.

Industrial wastewater treatment describes the processes used for treating wastewater that is produced by industries as an undesirable by-product. After treatment, the treated industrial wastewater may be reused or released to a sanitary sewer or to a surface water in the environment. Some industrial facilities generate wastewater that can be treated in sewage treatment plants. Most industrial processes, such as petroleum refineries, chemical and petrochemical plants have their own specialized facilities to treat their wastewaters so that the pollutant concentrations in the treated wastewater comply with the regulations regarding disposal of wastewaters into sewers or into rivers, lakes or oceans. This applies to industries that generate wastewater with high concentrations of organic matter, toxic pollutants or nutrients such as ammonia. Some industries install a pre-treatment system to remove some pollutants, and then discharge the partially treated wastewater to the municipal sewer system.

Sand filters are used as a step in the water treatment process of water purification.
Electrocoagulation (EC) is a technique used for wastewater treatment, wash water treatment, industrially processed water, and medical treatment. Electrocoagulation has become a rapidly growing area of wastewater treatment due to its ability to remove contaminants that are generally more difficult to remove by filtration or chemical treatment systems, such as emulsified oil, total petroleum hydrocarbons, refractory organics, suspended solids, and heavy metals. There are many brands of electrocoagulation devices available, and they can range in complexity from a simple anode and cathode to much more complex devices with control over electrode potentials, passivation, anode consumption, cell REDOX potentials as well as the introduction of ultrasonic sound, ultraviolet light and a range of gases and reactants to achieve so-called Advanced Oxidation Processes for refractory or recalcitrant organic substances.
Dissolved air flotation (DAF) is a water treatment process that clarifies wastewaters by the removal of suspended matter such as oil or solids. The removal is achieved by dissolving air in the water or wastewater under pressure and then releasing the air at atmospheric pressure in a flotation tank basin. The released air forms tiny bubbles which adhere to the suspended matter, causing the suspended matter to float to the surface of the water where it may then be removed by a skimming device.

Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.

Secondary treatment is the removal of biodegradable organic matter from sewage or similar kinds of wastewater. The aim is to achieve a certain degree of effluent quality in a sewage treatment plant suitable for the intended disposal or reuse option. A "primary treatment" step often precedes secondary treatment, whereby physical phase separation is used to remove settleable solids. During secondary treatment, biological processes are used to remove dissolved and suspended organic matter measured as biochemical oxygen demand (BOD). These processes are performed by microorganisms in a managed aerobic or anaerobic process depending on the treatment technology. Bacteria and protozoa consume biodegradable soluble organic contaminants while reproducing to form cells of biological solids. Secondary treatment is widely used in sewage treatment and is also applicable to many agricultural and industrial wastewaters.
A lamella clarifier or inclined plate settler (IPS) is a type of clarifier designed to remove particulates from liquids.

Clarifiers are settling tanks built with mechanical means for continuous removal of solids being deposited by sedimentation. A clarifier is generally used to remove solid particulates or suspended solids from liquid for clarification and/or thickening. Inside the clarifier, solid contaminants will settle down to the bottom of the tank where it is collected by a scraper mechanism. Concentrated impurities, discharged from the bottom of the tank, are known as sludge, while the particles that float to the surface of the liquid are called scum.
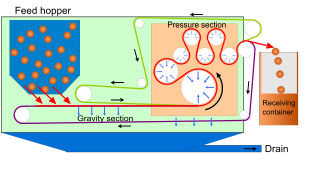
The belt filter is an industrial machine, used for solid/liquid separation processes, particularly the dewatering of sludges in the chemical industry, mining and water treatment. Belt filter presses are also used in the production of apple juice, cider and winemaking. The process of filtration is primarily obtained by passing a pair of filtering cloths and belts through a system of rollers. The system takes a sludge or slurry as a feed, and separates it into a filtrate and a solid cake.
Mixed liquor suspended solids (MLSS) is the concentration of suspended solids, in an aeration tank during the activated sludge process, which occurs during the treatment of waste water. The units MLSS is primarily measured in milligram per litre (mg/L), but for activated sludge its mostly measured in gram per litre [g/L] which is equal to kilogram per cubic metre [kg/m3]. Mixed liquor is a combination of raw or unsettled wastewater or pre-settled wastewater and activated sludge within an aeration tank. MLSS consists mostly of microorganisms and non-biodegradable suspended matter. MLSS is an important part of the activated sludge process to ensure that there is a sufficient quantity of active biomass available to consume the applied quantity of organic pollutant at any time. This is known as the food to microorganism ratio, more commonly notated as the F/M ratio. By maintaining this ratio at the appropriate level the biomass will consume high percentages of the food. This minimizes the loss of residual food in the treated effluent. In simple terms, the more the biomass consumes the lower the biochemical oxygen demand (BOD) will be in the discharge. It is important that MLSS removes COD and BOD in order to purify water for clean surface waters, and subsequently clean drinking water and hygiene. Raw sewage enters in the water treatment process with a concentration of sometimes several hundred mg/L of BOD. Upon being treated by screening, pre-settling, activated sludge processes or other methods of treatment, the concentration of BOD in water can be lowered to less than 2 mg/L, which is considered to be clean, safe to discharge to surface waters or to reuse water.

















