Related Research Articles

Glutamate carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction
A glutamyl-tRNA reductase (EC 1.2.1.70) is an enzyme that catalyzes the chemical reaction
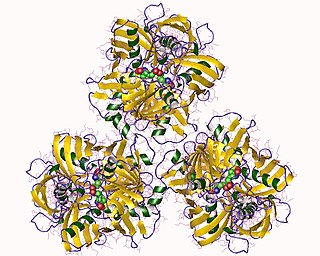
In enzymology, a diaminopimelate epimerase is an enzyme that catalyzes the chemical reaction

The enzyme diaminopimelate decarboxylase (EC 4.1.1.20) catalyzes the cleavage of carbon-carbon bonds in meso 2,6 diaminoheptanedioate to produce CO2 and L-lysine, the essential amino acid. It employs the cofactor pyridoxal phosphate, also known as PLP, which participates in numerous enzymatic transamination, decarboxylation and deamination reactions.
In enzymology, a UDP-N-acetylmuramoyl-L-alanyl-D-glutamate—L-lysine ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a UDP-N-acetylmuramoyl-tripeptide—D-alanyl-D-alanine ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyldiaminopimelate deacetylase (EC 3.5.1.47) is an enzyme that catalyzes the chemical reaction
Peptidoglycan glycosyltransferase is an enzyme used in the biosynthesis of peptidoglycan. It transfers a disaccharide-peptide from a donor substrate to synthesize a glycan chain.
In enzymology, an undecaprenyldiphospho-muramoylpentapeptide beta-N-acetylglucosaminyltransferase is an enzyme that catalyzes the chemical reaction

Subtilases are a family of subtilisin-like serine proteases. They appear to have independently and convergently evolved an Asp/Ser/His catalytic triad, like in the trypsin serine proteases. The structure of proteins in this family shows that they have an alpha/beta fold containing a 7-stranded parallel beta sheet.

Peptidoglycan binding domains have a general peptidoglycan binding function and a common core structure consisting of a closed, three-helical bundle with a left-handed twist. It is found at the N or C terminus of a variety of enzymes involved in bacterial cell wall degradation. Examples are:

In molecular biology, the CHAP domain is a region between 110 and 140 amino acids that is found in proteins from bacteria, bacteriophages, archaea and eukaryotes of the family Trypanosomidae. The domain is named after the acronym cysteine, histidine-dependent amidohydrolases/peptidases. Many of these proteins are uncharacterised, but it has been proposed that they may function mainly in peptidoglycan hydrolysis. The CHAP domain is found in a wide range of protein architectures; it is commonly associated with bacterial type SH3 domains and with several families of amidase domains. It has been suggested that CHAP domain containing proteins utilise a catalytic cysteine residue in a nucleophilic-attack mechanism.
Muramoylpentapeptide carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction.
Muramoyltetrapeptide carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Zinc D-Ala-D-Ala carboxypeptidase (EC 3.4.17.14, Zn2+ G peptidase, D-alanyl-D-alanine hydrolase, D-alanyl-D-alanine-cleaving carboxypeptidase, DD-carboxypeptidase, G enzyme, DD-carboxypeptidase-transpeptidase) is an enzyme. This enzyme catalyses the following chemical reaction

Ghk.
UDP-N-acetylmuramoyl-L-alanyl-D-glutamate—2,6-diaminopimelate ligase is an enzyme with systematic name UDP-N-acetylmuramoyl-L-alanyl-D-glutamate:meso-2,6-diaminoheptanedioate gamma-ligase (ADP-forming). This enzyme catalyses the following chemical reaction
Bacillus isolates have a variety of biotechnological applications.
Glutamyl endopeptidase I is a family of extracellular bacterial serine proteases. The proteases within this family have been identified in species of Staphylococcus, Bacillus, and Streptomyces, among others. The two former are more closely related, while the Streptomyces-type is treated as a separate family, glutamyl endopeptidase II.
References
- ↑ Arminjon F, Guinand M, Vacheron MJ, Michel G (March 1977). "Specificity profiles of the membrane-bound gamma-D-glutamyl-(L)meso-diaminopimelateendopeptidase and LD-carboxypeptidase from Bacillus sphaericus 9602". European Journal of Biochemistry. 73 (2): 557–65. doi:10.1111/j.1432-1033.1977.tb11351.x. PMID 849747.
- ↑ Garnier M, Vacheron MJ, Guinand M, Michel G (May 1985). "Purification and partial characterization of the extracellular gamma-D-glutamyl-(L)meso-diaminopimelate endopeptidase I, from Bacillus sphaericus NCTC 9602". European Journal of Biochemistry. 148 (3): 539–43. doi: 10.1111/j.1432-1033.1985.tb08873.x . PMID 3922755.
- ↑ Hourdou ML, Guinand M, Vacheron MJ, Michel G, Denoroy L, Duez C, Englebert S, Joris B, Weber G, Ghuysen JM (June 1993). "Characterization of the sporulation-related gamma-D-glutamyl-(L)meso-diaminopimelic-acid-hydrolysing peptidase I of Bacillus sphaericus NCTC 9602 as a member of the metallo(zinc) carboxypeptidase A family. Modular design of the protein". The Biochemical Journal. 292 (2): 563–70. doi:10.1042/bj2920563. PMC 1134247 . PMID 8503890.