Fluid replacement or fluid resuscitation is the medical practice of replenishing bodily fluid lost through sweating, bleeding, fluid shifts or other pathologic processes. Fluids can be replaced with oral rehydration therapy (drinking), intravenous therapy, rectally such as with a Murphy drip, or by hypodermoclysis, the direct injection of fluid into the subcutaneous tissue. Fluids administered by the oral and hypodermic routes are absorbed more slowly than those given intravenously.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.
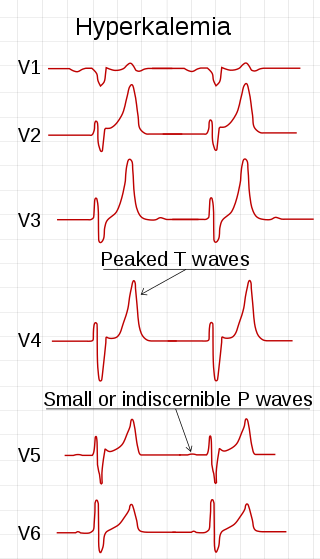
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.

Hypovolemic shock is a form of shock caused by severe hypovolemia. It can be caused by severe dehydration or blood loss. Hypovolemic shock is a medical emergency; if left untreated, the insufficient blood flow can cause damage to organs, leading to multiple organ failure.

Loop diuretics are pharmacological agents that primarily inhibit the Na-K-Cl cotransporter located on the luminal membrane of cells along the thick ascending limb of the loop of Henle. They are often used for the treatment of hypertension and edema secondary to congestive heart failure, liver cirrhosis, or chronic kidney disease. While thiazide diuretics are more effective in patients with normal kidney function, loop diuretics are more effective in patients with impaired kidney function.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat hypovolemia such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer's (LR), and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.
The anion gap is a value calculated from the results of multiple individual medical lab tests. It may be reported with the results of an electrolyte panel, which is often performed as part of a comprehensive metabolic panel.
Hypoaldosteronism is an endocrinological disorder characterized by decreased levels of the hormone aldosterone. Similarly, isolated hypoaldosteronism is the condition of having lowered aldosterone without corresponding changes in cortisol.

Metabolic alkalosis is an acid-base disorder in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
Hyperchloremic acidosis is a form of metabolic acidosis associated with a normal anion gap, a decrease in plasma bicarbonate concentration, and an increase in plasma chloride concentration. Although plasma anion gap is normal, this condition is often associated with an increased urine anion gap, due to the kidney's inability to secrete ammonia.

Renal tubular acidosis (RTA) is a medical condition that involves an accumulation of acid in the body due to a failure of the kidneys to appropriately acidify the urine. In renal physiology, when blood is filtered by the kidney, the filtrate passes through the tubules of the nephron, allowing for exchange of salts, acid equivalents, and other solutes before it drains into the bladder as urine. The metabolic acidosis that results from RTA may be caused either by insufficient secretion of hydrogen ions into the latter portions of the nephron or by failure to reabsorb sufficient bicarbonate ions from the filtrate in the early portion of the nephron. Although a metabolic acidosis also occurs in those with chronic kidney disease, the term RTA is reserved for individuals with poor urinary acidification in otherwise well-functioning kidneys. Several different types of RTA exist, which all have different syndromes and different causes. RTA is usually an incidental finding based on routine blood draws that show abnormal results. Clinically, patients may present with vague symptoms such as dehydration, mental status changes, or delayed growth in adolescents.
Metolazone is a thiazide-like diuretic marketed under the brand names Zytanix, Metoz, Zaroxolyn, and Mykrox. It is primarily used to treat congestive heart failure and high blood pressure. Metolazone indirectly decreases the amount of water reabsorbed into the bloodstream by the kidney, so that blood volume decreases and urine volume increases. This lowers blood pressure and prevents excess fluid accumulation in heart failure. Metolazone is sometimes used together with loop diuretics such as furosemide or bumetanide, but these highly effective combinations can lead to dehydration and electrolyte abnormalities.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.
Pseudohypoaldosteronism (PHA) is a condition that mimics hypoaldosteronism. Two major types of primary pseudohypoaldosteronism are recognized and these have major differences in etiology and presentation.
Normal anion gap acidosis is an acidosis that is not accompanied by an abnormally increased anion gap.

Nephrocalcinosis, once known as Albright's calcinosis after Fuller Albright, is a term originally used to describe the deposition of poorly soluble calcium salts in the renal parenchyma due to hyperparathyroidism. The term nephrocalcinosis is used to describe the deposition of both calcium oxalate and calcium phosphate. It may cause acute kidney injury. It is now more commonly used to describe diffuse, fine, renal parenchymal calcification in radiology. It is caused by multiple different conditions and is determined by progressive kidney dysfunction. These outlines eventually come together to form a dense mass. During its early stages, nephrocalcinosis is visible on x-ray, and appears as a fine granular mottling over the renal outlines. It is most commonly seen as an incidental finding with medullary sponge kidney on an abdominal x-ray. It may be severe enough to cause renal tubular acidosis or even end stage kidney disease, due to disruption of the kidney tissue by the deposited calcium salts.
A volume expander is a type of intravenous therapy that has the function of providing volume for the circulatory system. It may be used for fluid replacement or during surgery to prevent nausea and vomiting after surgery.