Related Research Articles

Bradykinin (BK) (Greek brady-, slow; -kinin, kīn(eîn) to move) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin consists of nine amino acids, and is a physiologically and pharmacologically active peptide of the kinin group of proteins.
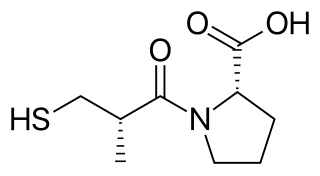
Captopril, sold under the brand name Capoten among others, is an angiotensin-converting enzyme (ACE) inhibitor used for the treatment of hypertension and some types of congestive heart failure. Captopril was the first oral ACE inhibitor found for the treatment of hypertension. It does not cause fatigue as associated with beta-blockers. Due to the adverse drug event of causing hyperkalemia, as seen with most ACE Inhibitors, the medication is usually paired with a diuretic.

Bothrops atrox — also known as the common lancehead, fer-de-lance, barba amarilla and mapepire balsain — is a highly venomous pit viper species found in the tropical lowlands of northern South America east of the Andes, as well as the Caribbean island of Trinidad. No subspecies are currently recognized.

Bothrops jararaca — known as the jararaca or yarara — is a highly venomous pit viper species endemic to South America in southern Brazil, Paraguay, and northern Argentina. The specific name, jararaca, is derived from the Tupi words yarará and ca, which mean "large snake". Within its geographic range, it is often abundant and is an important cause of snakebite. No subspecies are currently recognized.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.

Bothrops Insularis, commonly known as the Golden Lancehead, is a highly venomous pit viper species found exclusively on the Ilha da Queimada Grande, off the coast of São Paulo state, in Brazil. The species is named for the light yellowish-brown color of its underside and for its head shape that is characteristic of the genus Bothrops. No subspecies of Bothrops insularis are currently recognized. It is one of the most venomous snakes in Latin America.

In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction
Cerastocytin is a thrombin-like serine protease in snake venom.

Bothrops jararacussu, commonly known in English as the jararacussu, is a highly venomous pit viper species endemic to South America. It is one of the most dreaded snakes in South America and can grow up to 2.2 metres (7.2 ft).

Bothrops moojeni, commonly known in English as the Brazilian lancehead, is a species of venomous snake in the family Viperidae. It is a pit viper endemic to South America.

Teprotide is nonapeptide which has been isolated from the snake Bothrops jararaca. It is an angiotensin converting enzyme inhibitor (ACE inhibitor) which inhibits the conversion of angiotensin I to angiotensin II and may potentiate some of the pharmacological actions of bradykinin. It has a molecular formula of C53H76N14O12 and has been investigated as an antihypertension agent.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
Bothropasin is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin C is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin E is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin F is an enzyme. This enzyme catalyses the following chemical reaction
Ruberlysin is an enzyme. This enzyme catalyses the following chemical reaction
Bothrolysin is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin I is an enzyme. This enzyme catalyses the following chemical reaction
Trimerelysin II is an enzyme. This enzyme catalyses the following chemical reaction
References
- ↑ Mandelbaum, F.R., Reichl, A.P. and Assakura, M.T. (1976). "Some physical and biochemical characteristics of HF2, one of the hemorrhagic factors in the venom of Bothrops jararaca". In Ohsaka, A., Hayashi, K. and Sawai, Y. (ed.). Animal, Plant and Microbial Toxins. New York: Plenum Press. pp. 111–121.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Assakura MT, Reichl AP, Mandelbaum FR (1986). "Comparison of immunological, biochemical and biophysical properties of three hemorrhagic factors isolated from the venom of Bothrops jararaca (jararaca)". Toxicon. 24 (9): 943–6. doi:10.1016/0041-0101(86)90094-2. PMID 3810664.
- ↑ Paine MJ, Desmond HP, Theakston RD, Crampton JM (November 1992). "Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family". The Journal of Biological Chemistry. 267 (32): 22869–76. doi: 10.1016/S0021-9258(18)50027-2 . PMID 1385408.