Related Research Articles

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. Present in animals since early stages of evolution, in humans it plays roles in behavior that include social bonding, love, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during childbirth. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in maternal bonding and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.

The supraoptic nucleus (SON) is a nucleus of magnocellular neurosecretory cells in the hypothalamus of the mammalian brain. The nucleus is situated at the base of the brain, adjacent to the optic chiasm. In humans, the SON contains about 3,000 neurons.
The angiotensin II receptors, (ATR1) and (ATR2), are a class of G protein-coupled receptors with angiotensin II as their ligands. They are important in the renin–angiotensin system: they are responsible for the signal transduction of the vasoconstricting stimulus of the main effector hormone, angiotensin II.
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the N-terminus (beginning), of proteins or peptides. They are found in many organisms; in the cell, they are found in many organelles, in the cytosol, and as membrane proteins. Aminopeptidases are used in essential cellular functions, and are often zinc metalloenzymes, containing a zinc cofactor.

Insulin regulated aminopeptidase (IRAP) is a protein that in humans is encoded by the leucyl and cystinyl aminopeptidase (LNPEP) gene. IRAP is a type II transmembrane protein which belongs to the oxytocinase subfamily of M1 aminopeptidases, alongside ERAP1 and ERAP2. It is also known as oxytocinase, leucyl and cystinyl aminopeptidase, placental leucine aminopeptidase (P-LAP), cystinyl aminopeptidase (CAP), and vasopressinase. IRAP is expressed in different cell types, mainly located in specialized regulated endosomes that can be recruited to the cell surface upon cell type-specific receptor activation.

Carbetocin, sold under the brand names Pabal among others, is a medication used to prevent excessive bleeding after childbirth, particularly following Cesarean section. It appears to work as well as oxytocin. Due to it being less economical than other options, use is not recommended by NHS Scotland. It is given by injection into a vein or muscle.
In enzymology, a peptide-methionine (R)-S-oxide reductase (EC 1.8.4.12) is an enzyme that catalyzes the chemical reaction
The enzyme 1,5-anhydro-D-fructose dehydratase (EC 4.2.1.111) catalyzes the chemical reaction

Pregnancy-specific beta-1-glycoprotein 1 (PSBG-1) also known as CD66f, is a protein that in humans is encoded by the PSG1 gene and is a member of the carcinoembryonic antigen (CEA) gene family. Pregnancy-specific glycoproteins (PSGs) are a complex consisting of carbohydrate and protein, which is present in the mammalian body specifically during pregnancy. This glycoprotein is the most abundant protein found in the maternal bloodstream during the later stages of pregnancy and it is of vital importance in fetal development. The PSG functions primarily as an immunomodulator to protect the growing fetus.

Cathepsin H is a protein that in humans is encoded by the CTSH gene.
Alpha-1-microglobulin is a microglobulin, a small globular protein. It is found in all vertebrates, including humans, and is distributed in blood plasma and extravascular tissues of all organs. It is synthesized in most cells of the body, but mainly in the liver from a gene that codes for the alpha-1-microglobulin/bikunin precursor.
A uterotonic, also known as an oxytocic or ecbolic, is a type of medication used to induce contraction or greater tonicity of the uterus. Uterotonics are used both to induce labor and to reduce postpartum hemorrhage.

Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:

Melanocyte-inhibiting factor (also known as Pro-Leu-Gly-NH2, Melanostatin, MSH release–inhibiting hormone or MIF-1) is an endogenous peptide fragment derived from cleavage of the hormone oxytocin, but having generally different actions in the body. MIF-1 produces multiple effects, both blocking the effects of opioid receptor activation, while at the same time acting as a positive allosteric modulator of the D2 and D4 dopamine receptor subtypes, as well as inhibiting release of other neuropeptides such as alpha-MSH, and potentiating melatonin activity.
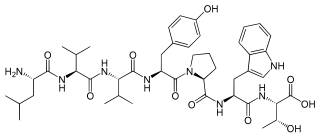
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Tynorphin is a synthetic opioid peptide which is a potent and competitive inhibitor of the enkephalinase class of enzymes which break down the endogenous enkephalin peptides. It specifically inactivates dipeptidyl aminopeptidase III (DPP3) with very high efficacy, but also inhibits neutral endopeptidase (NEP), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE) to a lesser extent. It has a pentapeptide structure with the amino acid sequence Val-Val-Tyr-Pro-Trp (VVYPW).

Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3. It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase, bacterial leucyl aminopeptidase, leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and, to a lesser extent, glutamyl aminopeptidase, as well as other aminopeptidases. It does not inhibit arginyl aminopeptidase. Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo. It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.
Hormones during pregnancy are the result of an intricate interaction between hormones generated by different glands and organs. The primary hormones involved comprise human chorionic gonadotropin (hCG), progesterone, estrogen, human placental lactogen (hPL), and oxytocin. Hormones are synthesized in certain organs, including the ovaries, placenta, and pituitary gland. These hormones have essential functions in pregnancy test, maintaining the uterine lining, fetal development, preventing premature labor, and the initiation and support of labor.
References
- 1 2 3 Tsujimoto M, Hattori A (2005). "The oxytocinase subfamily of M1 aminopeptidases". Biochim. Biophys. Acta. 1751 (1): 9–18. doi:10.1016/j.bbapap.2004.09.011. PMID 16054015.
- 1 2 Nomura S, Ito T, Yamamoto E, Sumigama S, Iwase A, Okada M, Shibata K, Ando H, Ino K, Kikkawa F, Mizutani S (2005). "Gene regulation and physiological function of placental leucine aminopeptidase/oxytocinase during pregnancy". Biochim. Biophys. Acta. 1751 (1): 19–25. doi:10.1016/j.bbapap.2005.04.006. PMID 15894523.
- 1 2 Mizutani S, Yokosawa H, Tomoda Y (1992). "Degradation of oxytocin by the human placenta: effect of selective inhibitors". Acta Endocrinol. 127 (1): 76–80. doi:10.1530/acta.0.1270076. PMID 1355623.
- ↑ Klimek, Marek (August 2005). "Comparative analysis of ACTH and oxytocinase plasma concentration during pregnancy". Neuro Endocrinology Letters. 26 (4): 337–341. ISSN 0172-780X. PMID 16136013.
- ↑ Meisenberg G, Simmons WH (1984). "Amastatin potentiates the behavioral effects of vasopressin and oxytocin in mice". Peptides. 5 (3): 535–9. doi:10.1016/0196-9781(84)90083-4. PMID 6540873. S2CID 3881661.
- ↑ Stancampiano R, Melis MR, Argiolas A (1991). "Proteolytic conversion of oxytocin by brain synaptic membranes: role of aminopeptidases and endopeptidases". Peptides. 12 (5): 1119–25. doi:10.1016/0196-9781(91)90068-z. PMID 1800950. S2CID 36706540.
- ↑ Itoh C, Watanabe M, Nagamatsu A, Soeda S, Kawarabayashi T, Shimeno H (1997). "Two molecular species of oxytocinase (L-cystine aminopeptidase) in human placenta: purification and characterization". Biol. Pharm. Bull. 20 (1): 20–4. doi: 10.1248/bpb.20.20 . PMID 9013800.