Phosphoric monoester hydrolases (or phosphomonoesterases) are enzymes that catalyse the hydrolysis of O-P bonds by nucleophilic attack of phosphorus by cysteine residues or coordinated metal ions.

Enzymes are macromolecular biological catalysts. Enzymes accelerate chemical reactions. The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron rich nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms to replace a leaving group; the positive or partially positive atom is referred to as an electrophile. The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule.

Phosphorus is a chemical element with symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram. With few exceptions, minerals containing phosphorus are in the maximally oxidized state as inorganic phosphate rocks.
They are categorized with the EC number 3.1.3.
Examples include:
- acid phosphatase
- alkaline phosphatase
- fructose-bisphosphatase
- glucose-6-phosphatase
- phosphofructokinase-2
- phosphoprotein phosphatase
- calcineurin
- 6-phytase
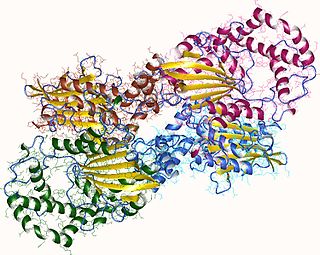
Acid phosphatase is a phosphatase, a type of enzyme, used to free attached phosphoryl groups from other molecules during digestion. It can be further classified as a phosphomonoesterase. Acid phosphatase is stored in lysosomes and functions when these fuse with endosomes, which are acidified while they function; therefore, it has an acid pH optimum. This enzyme is present in many animal and plant species.

Alkaline phosphatase or basic phosphatase is a homodimeric protein enzyme of 86 kilodaltons. Each monomer contains five cysteine residues, two zinc atoms, and one magnesium atom crucial to its catalytic function, and it is optimally active at alkaline pH environments. ALP has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of E. coli bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development within the skeleton. Due to its widespread prevalence in these areas, its concentration in the bloodstream is used by diagnosticians as a biomarker in helping determine diagnoses such as hepatitis or osteomalacia.

Fructose bisphosphatase (EC 3.1.3.11) is an enzyme that converts fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis and the Calvin cycle which are both anabolic pathways. Fructose bisphosphatase catalyses the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate, which is the reverse of the reaction which is catalysed by phosphofructokinase in glycolysis. These enzymes only catalyse the reaction in one direction each, and are regulated by metabolites such as fructose 2,6-bisphosphate so that high activity of one of the two enzymes is accompanied by low activity of the other. More specifically, fructose 2,6-bisphosphate allosterically inhibits fructose 1,6-bisphosphatase, but activates phosphofructokinase-I. Fructose 1,6-bisphosphatase is involved in many different metabolic pathways and found in most organisms. FBPase requires metal ions for catalysis (Mg2+ and Mn2+ being preferred) and the enzyme is potently inhibited by Li+.

