Related Research Articles

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.

Carboxyglutamic acid, is an uncommon amino acid introduced into proteins by a post-translational carboxylation of glutamic acid residues. This modification is found, for example, in clotting factors and other proteins of the coagulation cascade. This modification introduces an affinity for calcium ions. In the blood coagulation cascade, vitamin K is required to introduce γ-carboxylation of clotting factors II, VII, IX, X and protein Z.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is injected by unique fangs during a bite, whereas some species are also able to spit venom.

Coagulation factor VII is one of the proteins that causes blood to clot in the coagulation cascade, and in humans is coded for by the gene F7. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa, which in turn activates factor IX and factor X.

Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.

Russell's viper is a venomous snake in the family Viperidae native to the Indian subcontinent and one of the big four snakes in India. It was described in 1797 by George Shaw and Frederick Polydore Nodder, and named after Patrick Russell, who wrote about it in his 1796 work An account of Indian serpents, collected on the coast of Coromandel.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
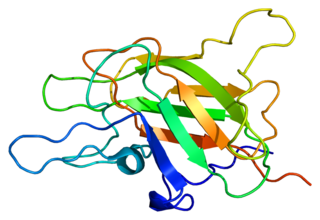
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.

Dilute Russell's viper venom time (dRVVT) is a laboratory test often used for detection of lupus anticoagulant (LA).
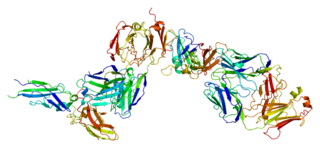
Tissue factor, also called platelet tissue factor, factor III, or CD142, is a protein encoded by the F3 gene, present in subendothelial tissue and leukocytes. Its role in the clotting process is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.
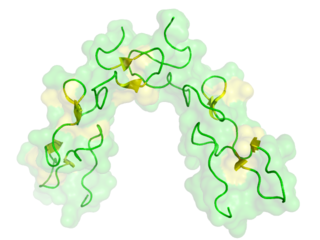
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.
Venom-induced consumption coagulopathy (VICC) is a medical condition caused by the effects of some snake and caterpillar venoms on the blood. Important coagulation factors are activated by the specific serine proteases in the venom and as they become exhausted, coagulopathy develops. Symptoms are consistent with uncontrolled bleeding. Diagnosis is made using blood tests that assess clotting ability along with recent history of envenomation. Treatment generally involves pressure dressing, confirmatory blood testing, and antivenom administration.
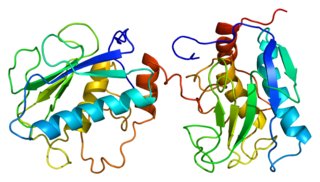
Stromelysin-1 also known as matrix metalloproteinase-3 (MMP-3) is an enzyme that in humans is encoded by the MMP3 gene. The MMP3 gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-3 has an estimated molecular weight of 54 kDa.

Bothrops moojeni, commonly known in English as the Brazilian lancehead, is a species of venomous snake in the family Viperidae. It is a pit viper endemic to South America.
Venombin A is an enzyme. This enzyme catalyses the following chemical reaction
Snake venom factor V activator is an enzyme. This enzyme catalyses the following chemical reaction
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
References
- ↑ Furie BC, Furie B (1976). Coagulant protein of Russell's viper venom. Methods in Enzymology. Vol. 45. pp. 191–205. doi:10.1016/s0076-6879(76)45019-x. PMID 1011991.
- ↑ Lindquist PA, Fujikawa K, Davie EW (March 1978). "Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom". The Journal of Biological Chemistry. 253 (6): 1902–9. PMID 632245.
- ↑ Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S (July 1992). "Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains". The Journal of Biological Chemistry. 267 (20): 14109–17. PMID 1629211.