Related Research Articles

Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals; that is, the way in which science is applied to the production of metals, and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist.

An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.

Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit. Ore is extracted from the earth through mining and treated or refined, often via smelting, to extract the valuable metals or minerals. The grade of ore refers to the concentration of the desired material it contains. The value of the metals or minerals a rock contains must be weighed against the cost of extraction to determine whether it is of sufficiently high grade to be worth mining, and is therefore considered an ore.

Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron.

Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal base behind. The reducing agent is commonly a fossil fuel source of carbon, such as coke—or, in earlier times, charcoal. The oxygen in the ore binds to carbon at high temperatures due to the lower potential energy of the bonds in carbon dioxide. Smelting most prominently takes place in a blast furnace to produce pig iron, which is converted into steel.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.

Redox is a type of chemical reaction in which the oxidation states of substrate change.
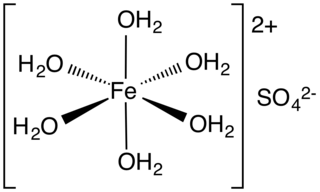
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

Sandpaper and glasspaper are names used for a type of coated abrasive that consists of sheets of paper or cloth with abrasive material glued to one face.

Ochre, or ocher in American English, is a natural clay earth pigment, a mixture of ferric oxide and varying amounts of clay and sand. It ranges in colour from yellow to deep orange or brown. It is also the name of the colours produced by this pigment, especially a light brownish-yellow. A variant of ochre containing a large amount of hematite, or dehydrated iron oxide, has a reddish tint known as "red ochre".

Industrial processes are procedures involving chemical, physical, electrical or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.

Greensand or green sand is a sand or sandstone which has a greenish color. This term is specifically applied to shallow marine sediment that contains noticeable quantities of rounded greenish grains. These grains are called glauconies and consist of a mixture of mixed-layer clay minerals, such as smectite and glauconite mica. Greensand is also loosely applied to any glauconitic sediment.
In soil science, agriculture and gardening, hardpan or soil pan is a dense layer of soil, usually found below the uppermost topsoil layer. There are different types of hardpan, all sharing the general characteristic of being a distinct soil layer that is largely impervious to water. Some hardpans are formed by deposits in the soil that fuse and bind the soil particles. These deposits can range from dissolved silica to matrices formed from iron oxides and calcium carbonate. Others are man-made, such as hardpan formed by compaction from repeated plowing, particularly with moldboard plows, or by heavy traffic or pollution.

Tamahagane (玉鋼) is a type of steel made in the Japanese tradition. The word tama means "precious". The word hagane means "steel". Tamahagane is used to make Japanese swords, knives, and other kinds of tools.

Polishing and buffing are finishing processes for smoothing a workpiece's surface using an abrasive and a work wheel or a leather strop. Technically polishing refers to processes that use an abrasive that is glued to the work wheel, while buffing uses a loose abrasive applied to the work wheel. Polishing is a more aggressive process while buffing is less harsh, which leads to a smoother, brighter finish. A common misconception is that a polished surface has a mirror bright finish, however most mirror bright finishes are actually buffed.

Ironsand, also known as iron-sand or iron sand, is a type of sand with heavy concentrations of iron. It is typically dark grey or blackish in colour.

Crushed stone or angular rock is a form of construction aggregate, typically produced by mining a suitable rock deposit and breaking the removed rock down to the desired size using crushers. It is distinct from naturally occurring gravel, which is produced by natural processes of weathering and erosion and typically has a more rounded shape.

Freshwater environmental quality parameters are those chemical, physical or biological parameters that can be used to characterise a freshwater body. Because almost all water bodies are dynamic in their composition, the relevant quality parameters are typically expressed as a range of expected concentrations.

Iron-rich sedimentary rocks are sedimentary rocks which contain 15 wt.% or more iron. However, most sedimentary rocks contain iron in varying degrees. The majority of these rocks were deposited during specific geologic time periods: The Precambrian, the early Paleozoic, and the middle to late Mesozoic. Overall, they make up a very small portion of the total sedimentary record.
References
- ↑ "silver sand - definition of silver sand in English from the Oxford dictionary". oxforddictionaries.com. Archived from the original on April 2, 2015.
- ↑ Sheat, Wilfrid G. (1965). Propagation of Trees Shrubs and Conifers. Macmillan. p. 17.