
Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized derivatives such as bacteriohopanepolyols (BHPs) and hopanoids covalently attached to lipid A.

Oleanane is a natural triterpenoid. It is commonly found in woody angiosperms and as a result is often used as an indicator of these plants in the fossil record. It is a member of the oleanoid series, which consists of pentacyclic triterpenoids where all rings are six-membered.

Cholestane is a saturated tetracyclic triterpene. This 27-carbon biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record. Presence of cholestane, its derivatives and related chemical compounds in environmental samples is commonly interpreted as an indicator of animal life and/or traces of O2, as animals are known for exclusively producing cholesterol, and thus has been used to draw evolutionary relationships between ancient organisms of unknown phylogenetic origin and modern metazoan taxa. Cholesterol is made in low abundance by other organisms (e.g., rhodophytes, land plants), but because these other organisms produce a variety of sterols it cannot be used as a conclusive indicator of any one taxon. It is often found in analysis of organic compounds in petroleum.
γ-Carotene (gamma-carotene) is a carotenoid, and is a biosynthetic intermediate for cyclized carotenoid synthesis in plants. It is formed from cyclization of lycopene by lycopene cyclase epsilon. Along with several other carotenoids, γ-carotene is a vitamer of vitamin A in herbivores and omnivores. Carotenoids with a cyclized, beta-ionone ring can be converted to vitamin A, also known as retinol, by the enzyme beta-carotene 15,15'-dioxygenase; however, the bioconversion of γ-carotene to retinol has not been well-characterized. γ-Carotene has tentatively been identified as a biomarker for green and purple sulfur bacteria in a sample from the 1.640 ± 0.003-Gyr-old Barney Creek Formation in Northern Australia which comprises marine sediments. Tentative discovery of γ-carotene in marine sediments implies a past euxinic environment, where water columns were anoxic and sulfidic. This is significant for reconstructing past oceanic conditions, but so far γ-carotene has only been potentially identified in the one measured sample.
Archaeol is composed of two phytanyl chains linked to the sn-2 and sn-3 positions of glycerol. As its phosphate ester, it is a common component of the membranes of archaea.

Isorenieratene /ˌaɪsoʊrəˈnɪərətiːn/ is a carotenoid light harvesting pigment produced exclusively by the genus Chlorobium. Chlorobium are the brown-colored strains of the family of green sulfur bacteria (Chlorobiaceae). Green sulfur bacteria are anaerobic photoautotrophic organisms meaning they perform photosynthesis in the absence of oxygen using hydrogen sulfide in the following reaction:
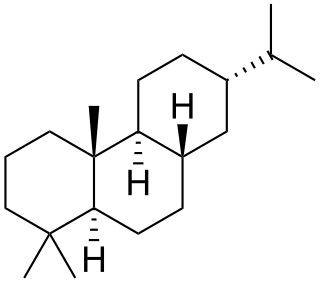
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.
Bacteriohopanepolyols (BHPs), bacteriohopanoids, or bacterial pentacyclic triterpenoids are commonly found in the lipid cell membranes of many bacteria. BHPs are frequently used as biomarkers in sedimentary rocks and can provide paleoecological information about ancient bacterial communities.

Dinosterol (4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol) is a 4α-methyl sterol that is produced by several genera of dinoflagellates and is rarely found in other classes of protists. The steroidal alkane, dinosterane, is the 'molecular fossil' of dinosterol, meaning that dinosterane has the same carbon skeleton as dinosterol, but lacks dinosterol's hydroxyl group and olefin functionality. As such, dinosterane is often used as a biomarker to identify the presence of dinoflagelletes in sediments.
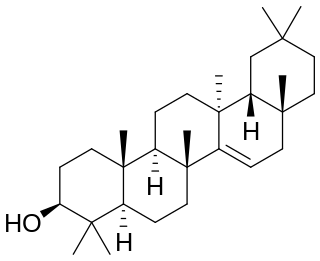
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.
Okenane, the diagenetic end product of okenone, is a biomarker for Chromatiaceae, the purple sulfur bacteria. These anoxygenic phototrophs use light for energy and sulfide as their electron donor and sulfur source. Discovery of okenane in marine sediments implies a past euxinic environment, where water columns were anoxic and sulfidic. This is potentially tremendously important for reconstructing past oceanic conditions, but so far okenane has only been identified in one Paleoproterozoic rock sample from Northern Australia.

24-Norcholestane, a steroid derivative, is used as a biomarker to constrain the source age of sediments and petroleum through the ratio between 24-norcholestane and 27-norcholestane, especially when used with other age diagnostic biomarkers, like oleanane. While the origins of this compound are still unknown, it is thought that they are derived from diatoms due to their identification in diatom rich sediments and environments. In addition, it was found that 24-norcholestane levels increased in correlation with diatom evolution. Another possible source of 24-norcholestane is from dinoflagellates, albeit to a much lower extent.
Crenarchaeol is a glycerol biphytanes glycerol tetraether (GDGT) biological membrane lipid. Together with archaeol, crenarcheol comprises a major component of archaeal membranes. Archaeal membranes are distinct from those of bacteria and eukaryotes because they contain isoprenoid GDGTs instead of diacyl lipids, which are found in the other domains. It has been proposed that GDGT membrane lipids are an adaptation to the high temperatures present in the environments that are home to extremophile archaea
Chlorobactane is the diagenetic product of an aromatic carotenoid produced uniquely by green-pigmented green sulfur bacteria (GSB) in the order Chlorobiales. Observed in organic matter as far back as the Paleoproterozoic, its identity as a diagnostic biomarker has been used to interpret ancient environments.

Isoarborinol is a triterpenoid ubiquitously produced by angiosperms and is thus considered a biomarker for higher plants. Though no isoarborinol-producing microbe has been identified, isoarborinol is also considered a possible biomarker for marine bacteria, as its diagenetic end product, arborane, has been found in ancient marine sediments that predate the rise of plants. Importantly, isoarborinol may represent the phylogenetic link between hopanols and sterols.

Gammacerane is a pentacyclic triterpene compound with the formula C30H52 and five six-membered rings. Its derivatives include tetrahymanol(Gammaceran-3β-ol)and so on. After millions of years of diagenesis, these derivatives became gammacerane can be used as biomarkers in petroleum to study the origin of petroleum.
Sulfur isotope biogeochemistry is the study of the distribution of sulfur isotopes in biological and geological materials. In addition to its common isotope, 32S, sulfur has three rare stable isotopes: 34S, 36S, and 33S. The distribution of these isotopes in the environment is controlled by many biochemical and physical processes, including biological metabolisms, mineral formation processes, and atmospheric chemistry. Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history.
Arborane is a class of pentacyclic triterpene consisting of organic compounds with four 6-membered rings and one 5-membered ring. Arboranes are thought to be derived from arborinols, a class of natural cyclic triterpenoids typically produced by flowering plants. Thus arboranes are used as a biomarker for angiosperms and cordaites. Arborane is a stereoisomer of a compound called fernane, the diagenetic product of fernene and fernenol. Because aborinol and fernenol have different biological sources, the ratio of arborane/fernane in a sample can be used to reconstruct a record for the relative abundances of different plants.

Lycopane (C40H82; 2,6,10,14,19,23,27,31-octamethyldotriacontane), a 40 carbon alkane isoprenoid, is a widely present biomarker that is often found in anoxic settings. It has been identified in anoxically deposited lacustrine sediments (such as the Messel formation and the Condor oil shale deposit). It has been found in sulfidic and anoxic hypersaline environments (such as the Sdom Formation). It has been widely identified in modern marine sediments, including the Peru upwelling zone, the Black Sea, and the Cariaco Trench. It has been found only rarely in crude oils.

Diplopterol is a triterpenoid molecule commonly produced by bacteria, ferns, and a few protozoans. This compound, classified as a member of the hopanoid family, is synthesized from triterpenoid precursor squalene. It is generally believed that hopanoids serve a similar function in bacteria as that of sterols in eukaryotes, which involves modulating membrane fluidity. Diplopterol serves as a useful biomarker for prokaryotic life, along with oxygen content at the time of sediment deposition.














