
Entropy is a scientific concept that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or phase changes such as melting and boiling. A reaction may release or absorb energy, and a phase change may do the same. Thermochemistry focuses on the energy exchange between a system and its surroundings in the form of heat. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.

A thermometer is a device that measures temperature or temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.

Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.

A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. It is one of the measurement devices used in the study of thermodynamics, chemistry, and biochemistry.

An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions.

The zeroth law of thermodynamics is one of the four principal laws of thermodynamics. It provides an independent definition of temperature without reference to entropy, which is defined in the second law. The law was established by Ralph H. Fowler in the 1930s, long after the first, second, and third laws had been widely recognized.
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, not only is there an absence of macroscopic change, but there is an “absence of any tendency toward change on a macroscopic scale.”

In thermodynamics, a reversible process is a process, involving a system and its surroundings, whose direction can be reversed by infinitesimal changes in some properties of the surroundings, such as pressure or temperature.

Thermodynamics is expressed by a mathematical framework of thermodynamic equations which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics.
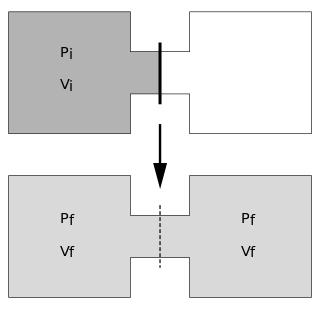
The Joule expansion is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container, with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container.

Classical thermodynamics considers three main kinds of thermodynamic processes: (1) changes in a system, (2) cycles in a system, and (3) flow processes.
In classical thermodynamics, entropy is a property of a thermodynamic system that expresses the direction or outcome of spontaneous changes in the system. The term was introduced by Rudolf Clausius in the mid-19th century to explain the relationship of the internal energy that is available or unavailable for transformations in form of heat and work. Entropy predicts that certain processes are irreversible or impossible, despite not violating the conservation of energy. The definition of entropy is central to the establishment of the second law of thermodynamics, which states that the entropy of isolated systems cannot decrease with time, as they always tend to arrive at a state of thermodynamic equilibrium, where the entropy is highest. Entropy is therefore also considered to be a measure of disorder in the system.

A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.

In thermodynamics, heat is the thermal energy transferred between systems due to a temperature difference. In colloquial use, heat sometimes refers to thermal energy itself. Thermal energy is the kinetic energy of vibrating and colliding atoms in a substance.

Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.

In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law. The physical region covered by a system may or may not coincide with a control volume used to analyze the system.
Scale of temperature is a methodology of calibrating the physical quantity temperature in metrology. Empirical scales measure temperature in relation to convenient and stable parameters or reference points, such as the freezing and boiling point of water. Absolute temperature is based on thermodynamic principles: using the lowest possible temperature as the zero point, and selecting a convenient incremental unit.






















