A berry is a small, pulpy, and often edible fruit. Typically, berries are juicy, rounded, brightly colored, sweet, sour or tart, and do not have a stone or pit, although many pips or seeds may be present. Common examples of berries in the culinary sense are strawberries, raspberries, blueberries, blackberries, white currants, blackcurrants, and redcurrants. In Britain, soft fruit is a horticultural term for such fruits.

Rubus idaeus is a red-fruited species of Rubus native to Europe and northern Asia and commonly cultivated in other temperate regions.
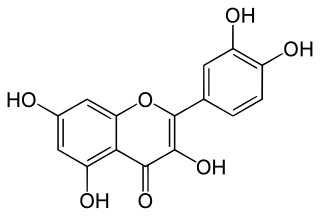
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

The garden strawberry is a widely grown hybrid species of the genus Fragaria, collectively known as the strawberries, which are cultivated worldwide for their fruit. The fruit is widely appreciated for its characteristic aroma, bright red color, juicy texture, and sweetness. It is consumed in large quantities, either fresh or in such prepared foods as jam, juice, pies, ice cream, milkshakes, and chocolates. Artificial strawberry flavorings and aromas are also widely used in products such as candy, soap, lip gloss, perfume, and many others.

Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

A polyphenol antioxidant is a hypothetized type of antioxidant, in which each instance would contain a polyphenolic substructure; such instances which have been studied in vitro. Numbering over 4,000 distinct chemical structures, such polyphenols may have antioxidant activity {{{1}}} in vitro (although they are unlikely to be antioxidants in vivo). Hypothetically, they may affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation, although high-quality clinical research has not confirmed any of these possible effects in humans as of 2020.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Miquelianin is a flavonol glucuronide, a type of phenolic compound present in wine, in species of St John's wort, like Hypericum hirsutum, in Nelumbo nucifera or in green beans.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.

Lambertianin C is an ellagitannin.

Sanguiin H-6 is an ellagitannin.
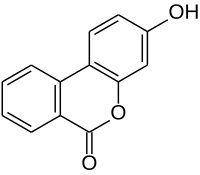
Urolithins are microflora metabolites of dietary ellagic acid derivatives, such as ellagitannins. They are produced in the gut, and found in the urine in the form of urolithin B glucuronide after absorption of ellagitannins-containing foods, such as pomegranate. During intestinal metabolism by bacteria, ellagitannins and punicalagins are converted to urolithins, which have unknown biological activity in vivo.

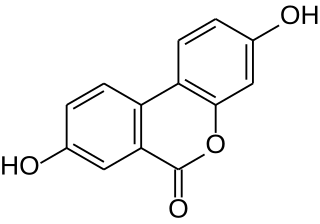
Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, walnuts, and others.

Tellimagrandin I is an ellagitannin found in plants, such as Cornus canadensis, Eucalyptus globulus, Melaleuca styphelioides, Rosa rugosa, and walnut. It is composed of two galloyl and one hexahydroxydiphenyl groups bound to a glucose residue. It differs from Tellimagrandin II only by a hydroxyl group instead of a third galloyl group. It is also structurally similar to punigluconin and pedunculagin, two more ellagitannin monomers.