
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees.

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled.

Respiratory syncytial virus (RSV), also called human respiratory syncytial virus (hRSV) and human orthopneumovirus, is a common, contagious virus that causes infections of the respiratory tract. It is a negative-sense, single-stranded RNA virus. Its name is derived from the large cells known as syncytia that form when infected cells fuse.

Human metapneumovirus (HMPV) is a negative-sense single-stranded RNA virus of the family Pneumoviridae and is closely related to the Avian metapneumovirus (AMPV) subgroup C. It was isolated for the first time in 2001 in the Netherlands by using the RAP-PCR technique for identification of unknown viruses growing in cultured cells. It is the second most common cause after Respiratory syncytial virus (RSV) of lower respiratory infection in young children.
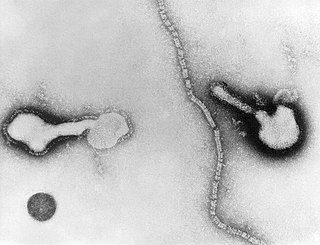
Human parainfluenza viruses (HPIVs) are the viruses that cause human parainfluenza. HPIVs are a paraphyletic group of four distinct single-stranded RNA viruses belonging to the Paramyxoviridae family. These viruses are closely associated with both human and veterinary disease. Virions are approximately 150–250 nm in size and contain negative sense RNA with a genome encompassing about 15,000 nucleotides.
Gregory Antone Prince is an American pathology researcher, businessman, author, social critic, and historian of the Latter Day Saint movement.

Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza and COVID infections used in Russia and China. The drug is manufactured by Pharmstandard. It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.
Palivizumab, sold under the brand name Synagis, is a monoclonal antibody produced by recombinant DNA technology used to prevent severe disease caused by respiratory syncytial virus (RSV) infections. It is recommended for infants at high-risk for RSV due to conditions such as prematurity or other medical problems including heart or lung diseases.
Respiratory syncytial virus G protein is a protein produced by respiratory syncytial virus.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.
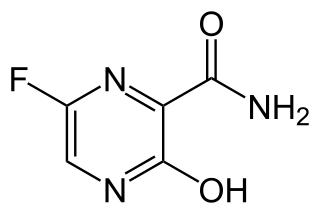
Favipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan. It is also being studied to treat a number of other viral infections, including SARS-CoV-2. Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.

Pneumoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Humans, cattle, and rodents serve as natural hosts. Respiratory tract infections are associated with member viruses such as human respiratory syncytial virus. There are five species in the family which are divided between the genera Metapneumovirus and Orthopneumovirus. The family used to be considered as a sub-family of Paramyxoviridae, but has been reclassified as of 2016.

Pimodivir is an antiviral drug which was developed as a treatment for influenza. It acts as an inhibitor of influenza virus polymerase basic protein 2, and has shown promising results in Phase II clinical trials. However, in late 2021, Janssen announced that the clinical development of pimidivir had been halted due to lack of benefit over standard of care.
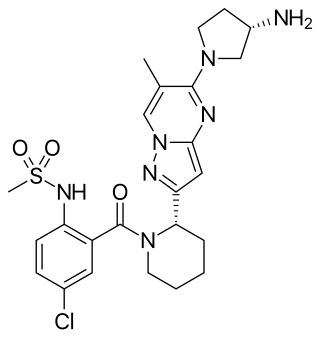
Presatovir (GS-5806) is an antiviral drug which was developed as a treatment for respiratory syncytial virus. It acts as a fusion inhibitor, and has shown promising results in Phase II clinical trials.
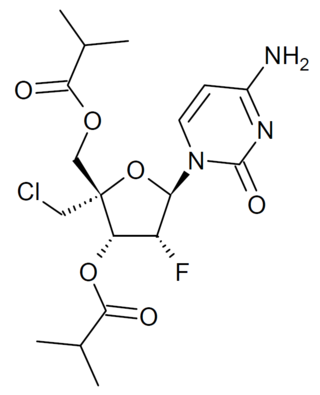
Lumicitabine (ALS-8176) is an antiviral drug which was developed as a treatment for respiratory syncytial virus (RSV) and human metapneumovirus (hMPV). It acts as an RNA polymerase inhibitor. While it showed promise in early clinical trials, poor results in Phase IIb trials led to it being discontinued from development for treatment of RSV. Research continues to determine whether it may be useful for the treatment of diseases caused by other RNA viruses, and it has been found to show activity against Nipah virus.
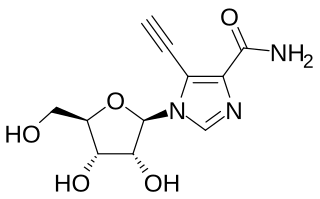
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.
A respiratory syncytial virus vaccine is a vaccine which prevents infection by respiratory syncytial virus. As of 2021, no licensed vaccine against RSV exists.
Jason S. McLellan is a structural biologist, professor in the Department of Molecular Biosciences and Robert A. Welch Chair in Chemistry at The University of Texas at Austin who specializes in understanding the structure and function of viral proteins, including those of coronaviruses. His research focuses on applying structural information to the rational design of vaccines and other therapies for viruses, including SARS-CoV-2, the novel coronavirus that causes COVID-19. McLellan and his team collaborated with researchers at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center to design a stabilized version of the SARS-CoV-2 spike protein, which biotechnology company Moderna used as the basis for the vaccine mRNA-1273, the first COVID-19 vaccine candidate to enter phase I clinical trials in the U.S. At least three other vaccines use this modified spike protein: those from Pfizer and BioNTech; Johnson & Johnson and Janssen Pharmaceuticals; and Novavax.
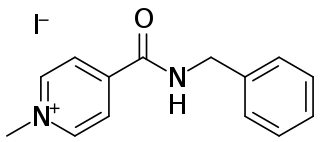
Enisamium iodide is a derivative of isonicotinic acid. Based on its systematic chemical name of N-benzyl-1-methylpyridin-1-ium-4-carboxamide iodide, the shortened name carbabenzpiride is sometimes used. Enisamium iodide is a registered antiviral drug sold in Ukraine, Kazakhstan, Mongolia, Belarus, and other Eastern European countries under the trade names Amizon, Amizon Max, Amizonchik.














