Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL has been associated with the progression of atherosclerosis.

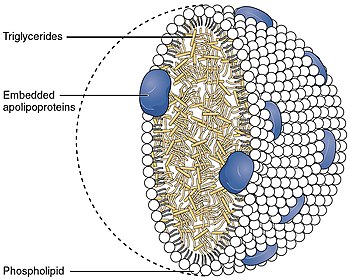
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role.
Very-low-density lipoprotein (VLDL), density relative to extracellular water, is a type of lipoprotein made by the liver. VLDL is one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. VLDL is assembled in the liver from triglycerides, cholesterol, and apolipoproteins. VLDL is converted in the bloodstream to low-density lipoprotein (LDL) and intermediate-density lipoprotein (IDL). VLDL particles have a diameter of 30–80 nanometers (nm). VLDL transports endogenous products, whereas chylomicrons transport exogenous (dietary) products. In the early 2010s both the lipid composition and protein composition of this lipoprotein were characterised in great detail.

Chylomicrons, also known as ultra low-density lipoproteins (ULDL), are lipoprotein particles that consist of triglycerides (85–92%), phospholipids (6–12%), cholesterol (1–3%), and proteins (1–2%). They transport dietary lipids, such as fats and cholesterol, from the intestines to other locations in the body, within the water-based solution of the bloodstream. ULDLs are one of the five major groups lipoproteins are divided into based on their density. A protein specific to chylomicrons is ApoB48.

Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids in blood, cerebrospinal fluid and lymph.
Hyperlipidemia is abnormally high levels of any or all lipids or lipoproteins in the blood. The term hyperlipidemia refers to the laboratory finding itself and is also used as an umbrella term covering any of various acquired or genetic disorders that result in that finding. Hyperlipidemia represents a subset of dyslipidemia and a superset of hypercholesterolemia. Hyperlipidemia is usually chronic and requires ongoing medication to control blood lipid levels.

Apolipoprotein E (Apo-E) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer's disease and cardiovascular diseases. It is encoded in humans by the gene APOE.

Apolipoprotein B (ApoB) is a protein that in humans is encoded by the APOB gene. Its measurement is commonly used to detect risk of atherosclerotic cardiovascular disease.

Apolipoprotein C-II, or apolipoprotein C2 is a protein that in humans is encoded by the APOC2 gene.

Apolipoprotein C-I is a protein component of lipoproteins that in humans is encoded by the APOC1 gene.
Lecithin cholesterol acyltransferase deficiency is a disorder of lipoprotein metabolism. The disease has two forms: Familial LCAT deficiency, in which there is complete LCAT deficiency, and Fish-eye disease, in which there is a partial deficiency.

Apolipoprotein AI(Apo-AI) is a protein that in humans is encoded by the APOA1 gene. As the major component of HDL particles, it has a specific role in lipid metabolism.

Apolipoprotein C-IV, also known as apolipoprotein C4, is a protein that in humans is encoded by the APOC4 gene.
Apolipoprotein D (ApoD) is a protein that in humans is encoded by the APOD gene. Unlike other lipoproteins, which are mainly produced in the liver, apolipoprotein D is mainly produced in the brain and testes. It is a 29 kDa glycoprotein discovered in 1963 as a component of the high-density lipoprotein (HDL) fraction of human plasma. It is the major component of human mammary cyst fluid. The human gene encoding it was cloned in 1986 and the deduced protein sequence revealed that ApoD is a member of the lipocalin family, small hydrophobic molecule transporters. ApoD is 169 amino acids long, including a secretion peptide signal of 20 amino acids. It contains two glycosylation sites and the molecular weight of the mature protein varies from 20 to 32 kDa.

Hepatic lipase (HL), also called hepatic triglyceride lipase (HTGL) or LIPC (for "lipase, hepatic"), is a form of lipase, catalyzing the hydrolysis of triacylglyceride. Hepatic lipase is coded by chromosome 15 and its gene is also often referred to as HTGL or LIPC. Hepatic lipase is expressed mainly in liver cells, known as hepatocytes, and endothelial cells of the liver. The hepatic lipase can either remain attached to the liver or can unbind from the liver endothelial cells and is free to enter the body's circulation system. When bound on the endothelial cells of the liver, it is often found bound to heparan sulfate proteoglycans (HSPG), keeping HL inactive and unable to bind to HDL (high-density lipoprotein) or IDL (intermediate-density lipoprotein). When it is free in the bloodstream, however, it is found associated with HDL to maintain it inactive. This is because the triacylglycerides in HDL serve as a substrate, but the lipoprotein contains proteins around the triacylglycerides that can prevent the triacylglycerides from being broken down by HL.

Apolipoprotein A-V is a protein that in humans is encoded by the APOA5 gene on chromosome 11. It is significantly expressed in liver. The protein encoded by this gene is an apolipoprotein and an important determinant of plasma triglyceride levels, a major risk factor for coronary artery disease. It is a component of several lipoprotein fractions including VLDL, HDL, chylomicrons. It is believed that apoA-V affects lipoprotein metabolism by interacting with LDL-R gene family receptors. Considering its association with lipoprotein levels, APOA5 is implicated in metabolic syndrome. The APOA5 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

Apolipoprotein A-II is a protein that in humans is encoded by the APOA2 gene. It is the second most abundant protein of the high density lipoprotein particles. The protein is found in plasma as a monomer, homodimer, or heterodimer with apolipoprotein D. ApoA-II regulates many steps in HDL metabolism, and its role in coronary heart disease is unclear. Remarkably, defects in this gene may result in apolipoprotein A-II deficiency or hypercholesterolemia.

Apolipoprotein A-IV is plasma protein that is the product of the human gene APOA4.

Microsomal triglyceride transfer protein large subunit is a protein that in humans is encoded by the MTTP, also known as MTP, gene.
Apolipoprotein M is an apolipoprotein and member of the lipocalin protein family that in humans is encoded by the APOM gene. It is found associated with high density lipoproteins and to a lesser extent with low density lipoproteins and triglyceride-rich lipoproteins. The encoded protein is secreted through the plasma membrane but remains membrane-bound, where it is involved in lipid transport. Two transcript variants encoding two different isoforms have been found for this gene, but only one of them has been fully characterized. It lacks an external amphipathic motif and is uniquely secreted to plasma without cleavage of its terminal signal peptide. The average molecular weight is 21253 Da, and the monoisotopic molecular weight is 21239 Da.