Related Research Articles

In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and right in the retroperitoneal space, and in adult humans are about 12 centimetres in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder.

Creatinine is a breakdown product of creatine phosphate from muscle and protein metabolism. It is released at a constant rate by the body.
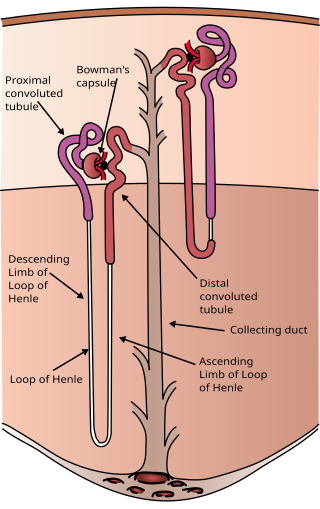
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the podocyte foot processes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged ; first with the interstitial fluid outside the tubules, and then into the plasma in the adjacent peritubular capillaries through the endothelial cells lining that capillary. This process regulates the volume of body fluid as well as levels of many body substances. At the end of the tubule, the remaining fluid—urine—exits: it is composed of water, metabolic waste, and toxins.

The renin-angiotensin system (RAS), or renin-angiotensin-aldosterone system (RAAS), is a hormone system that regulates blood pressure, fluid, and electrolyte balance, and systemic vascular resistance.

Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the adrenal cortex to promote sodium retention by the kidneys.

Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Uremia is the condition of having high levels of urea in the blood. Urea is one of the primary components of urine. It can be defined as an excess in the blood of amino acid and protein metabolism end products, such as urea and creatinine, which would normally be excreted in the urine. Uremic syndrome can be defined as the terminal clinical manifestation of kidney failure. It is the signs, symptoms and results from laboratory tests which result from inadequate excretory, regulatory, and endocrine function of the kidneys. Both uremia and uremic syndrome have been used interchangeably to denote a very high plasma urea concentration that is the result of renal failure. The former denotation will be used for the rest of the article.

Renal functions include maintaining an acid–base balance; regulating fluid balance; regulating sodium, potassium, and other electrolytes; clearing toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Assessment of kidney function occurs in different ways, using the presence of symptoms and signs, as well as measurements using urine tests, blood tests, and medical imaging.

In the kidney, the macula densa is an area of closely packed specialized cells lining the wall of the distal tubule where it touches the glomerulus. Specifically, the macula densa is found in the terminal portion of the distal straight tubule, after which the distal convoluted tubule begins.

Loop diuretics are pharmacological agents that primarily inhibit the Na-K-Cl cotransporter located on the luminal membrane of cells along the thick ascending limb of the loop of Henle. They are often used for the treatment of hypertension and edema secondary to congestive heart failure, liver cirrhosis, or chronic kidney disease. While thiazide diuretics are more effective in patients with normal kidney function, loop diuretics are more effective in patients with impaired kidney function.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.

The efferent arterioles are blood vessels that are part of the urinary tract of organisms. Efferent means "outgoing", in this case meaning carrying blood out away from the glomerulus. The efferent arterioles form a convergence of the capillaries of the glomerulus, and carry blood away from the glomerulus that has already been filtered. They play an important role in maintaining the glomerular filtration rate despite fluctuations in blood pressure.

In renal physiology, reabsorption, more specifically tubular reabsorption, is the process by which the nephron removes water and solutes from the tubular fluid (pre-urine) and returns them to the circulating blood. It is called reabsorption (and not absorption) because these substances have already been absorbed once (particularly in the intestines) and the body is reclaiming them from a postglomerular fluid stream that is on its way to becoming urine (that is, they will soon be lost to the urine unless they are reabsorbed from the tubule into the peritubular capillaries. This happens as a result of sodium transport from the lumen into the blood by the Na+/K+ATPase in the basolateral membrane of the epithelial cells. Thus, the glomerular filtrate becomes more concentrated, which is one of the steps in forming urine. Nephrons are divided into five segments, with different segments responsible for reabsorbing different substances. Reabsorption allows many useful solutes (primarily glucose and amino acids), salts and water that have passed through Bowman's capsule, to return to the circulation. These solutes are reabsorbed isotonically, in that the osmotic potential of the fluid leaving the proximal convoluted tubule is the same as that of the initial glomerular filtrate. However, glucose, amino acids, inorganic phosphate, and some other solutes are reabsorbed via secondary active transport through cotransport channels driven by the sodium gradient.
In the physiology of the kidney, tubuloglomerular feedback (TGF) is a feedback system inside the kidneys. Within each nephron, information from the renal tubules is signaled to the glomerulus. Tubuloglomerular feedback is one of several mechanisms the kidney uses to regulate glomerular filtration rate (GFR). It involves the concept of purinergic signaling, in which an increased distal tubular sodium chloride concentration causes a basolateral release of adenosine from the macula densa cells. This initiates a cascade of events that ultimately brings GFR to an appropriate level.
In medicine, the urea-to-creatinine ratio (UCR), known in the United States as BUN-to-creatinine ratio, is the ratio of the blood levels of urea (BUN) (mmol/L) and creatinine (Cr) (μmol/L). BUN only reflects the nitrogen content of urea and urea measurement reflects the whole of the molecule, urea is just over twice BUN. In the United States, both quantities are given in mg/dL The ratio may be used to determine the cause of acute kidney injury or dehydration.
The fractional excretion of sodium (FENa) is the percentage of the sodium filtered by the kidney which is excreted in the urine. It is measured in terms of plasma and urine sodium, rather than by the interpretation of urinary sodium concentration alone, as urinary sodium concentrations can vary with water reabsorption. Therefore, the urinary and plasma concentrations of sodium must be compared to get an accurate picture of kidney clearance. In clinical use, the fractional excretion of sodium can be calculated as part of the evaluation of acute kidney failure in order to determine if hypovolemia or decreased effective circulating plasma volume is a contributor to the kidney failure.
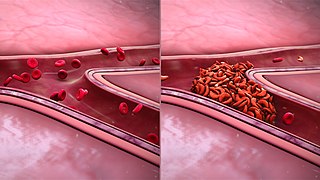
Sickle cell nephropathy is a type of kidney disease associated with sickle cell disease which causes kidney complications as a result of sickling of red blood cells in the small blood vessels. The hypertonic and relatively hypoxic environment of the renal medulla, coupled with the slow blood flow in the vasa recta, favors sickling of red blood cells, with resultant local infarction. Functional tubule defects in patients with sickle cell disease are likely the result of partial ischemic injury to the renal tubules.

The chronic kidney disease of the cat – also called chronic renal insufficiency or chronic renal failure (CRF) in the older literature – is an incurable, progressive disease characterized by a gradual decrease in the nephrons and thus to a decreasing function (insufficiency) of the kidneys. It is one of the most common causes of death in older domestic cats. In current literature, the term "kidney disease" is preferred to the term "renal insufficiency" because the disease initially progresses without any measurable decline in kidney function. Due to the different type of diet and the resulting metabolic peculiarities, the clinical picture and treatment sometimes differ significantly from chronic renal failure in humans.
References
- 1 2 3 Kumar, Vinay; Fausto, Nelson; Fausto, Nelso; Robbins, Stanley L.; Abbas, Abul K.; Cotran, Ramzi S. (2005). Robbins and Cotran Pathologic Basis of Disease (7th ed.). Philadelphia, Pa.: Elsevier Saunders. pp. 960, 1012. ISBN 0-7216-0187-1.
- ↑ Tyagi, Alka; Aeddula, Narothama R. (2022), "Azotemia", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30844172 , retrieved 2023-03-02
- 1 2 3 4 5 Goljan, Edward F. (2007). Rapid Review Pathology (2nd ed.). Mosby. pp. 396–398. ISBN 978-0-323-04414-1.
- ↑ Blantz, Roland C. (1998-02-01). "Pathophysiology of pre-renal azotemia". Kidney International. 53 (2): 512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x . ISSN 0085-2538. PMID 9461116.
- ↑ Tyagi, Alka; Aeddula, Narothama R. (2022), "Azotemia", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30844172 , retrieved 2022-06-15
- ↑ "Types of Azotemia". AyurvedicCure.com. Archived from the original on 2016-03-16. Retrieved 2010-10-20.