An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals. It is thought that an HIV vaccine could either induce an immune response against HIV or consist of preformed antibodies against HIV.

H5N1 clinical trials are clinical trials concerning H5N1 vaccines, which are intended to provide immunization to influenza A virus subtype H5N1. They are intended to discover pharmacological effects and identify any adverse reactions the vaccines may achieve in humans.
The STEP Study was a Phase IIb clinical trial intended to study the efficacy of an experimental HIV vaccine based on a human adenovirus 5 (HAdV-5) vector. The study was conducted in North and South America, the Caribbean, and Australia. A related study using the same experimental vaccine was conducted simultaneously in South Africa. These trials were co-sponsored by Merck, the HIV Vaccine Trials Network (HVTN), and the National Institute of Allergy and Infectious Diseases (NIAID), and had an Oversight Committee consisting of representatives from these three organizations. In South Africa the trial was overseen by the South African AIDS Vaccine Initiative.
HVTN 505 is a clinical trial testing an HIV vaccine regimen on research participants. The trial is conducted by the HIV Vaccine Trials Network and sponsored by the National Institute of Allergy and Infectious Diseases. Vaccinations were stopped in April 2013 due to initial results showing that the vaccine was ineffective in preventing HIV infections and lowering viral load among those participants who had become infected with HIV. All study participants will continue to be monitored for safety and any long-term effects.
The species Sudan ebolavirus is a virological taxon included in the genus Ebolavirus, family Filoviridae, order Mononegavirales. The species has a single virus member, Sudan virus (SUDV). The members of the species are called Sudan ebolaviruses. It was discovered in 1977 and causes Ebola clinically indistinguishable from the ebola Zaire strain, but is less transmissible than it. Unlike with ebola Zaire there is no vaccine available.

Marburg virus (MARV) is a hemorrhagic fever virus of the Filoviridae family of viruses and a member of the species Marburg marburgvirus, genus Marburgvirus. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen. In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group.

Bavarian Nordic A/S is a fully integrated biotechnology company focused on the development, manufacturing and commercialization of vaccines. The company is headquartered in Hellerup, Denmark, with manufacturing facilities in Kvistgård, Denmark and Thörishaus near Bern, Switzerland. The company has research and development facilities in Martinsried, Germany and San Diego, California, as well as offices in Zug, Switzerland, and Morrisville, North Carolina. The company uses viral vectors and virus-like particles in its research and development.

Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

There is a cure for the Ebola virus disease that is currently approved for market the US government has inventory in the Strategic National Stockpile. For past and current Ebola epidemics, treatment has been primarily supportive in nature.
Sir Adrian Vivian Sinton Hill, is a British-Irish vaccinologist who is Director of the Jenner Institute and Lakshmi Mittal and Family Professor of Vaccinology at the University of Oxford, an honorary Consultant Physician in Infectious Diseases, and Fellow of Magdalen College, Oxford. Hill is a leader in the field of malaria vaccine development and was a co-leader of the research team which produced the Oxford–AstraZeneca COVID-19 vaccine, along with Professor Sarah Gilbert of the Jenner Institute and Professor Andrew Pollard of the Oxford Vaccine Group.

Julie E. Ledgerwood is an American allergist and immunologist, who is the chief medical officer and chief of the Clinical Trials Program at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health in Bethesda, Maryland. She is a Doctor of Osteopathic Medicine.
A Zika virus vaccine is designed to prevent the symptoms and complications of Zika virus infection in humans. As Zika virus infection of pregnant women may result in congenital defects in the newborn, the vaccine will attempt to protect against congenital Zika syndrome during the current or any future outbreak. As of April 2019, no vaccines have been approved for clinical use, however a number of vaccines are currently in clinical trials. The goal of a Zika virus vaccine is to produce specific antibodies against the Zika virus to prevent infection and severe disease. The challenges in developing a safe and effective vaccine include limiting side effects such as Guillain-Barré syndrome, a potential consequence of Zika virus infection. Additionally, as dengue virus is closely related to Zika virus, the vaccine needs to minimize the possibility of antibody-dependent enhancement of dengue virus infection.

The 2018 Équateur province Ebola outbreak occurred in the north-west of the Democratic Republic of the Congo (DRC) from May to July 2018. It was contained entirely within Équateur province, and was the first time that vaccination with the rVSV-ZEBOV Ebola vaccine had been attempted in the early stages of an Ebola outbreak, with a total of 3,481 people vaccinated. It was the ninth recorded Ebola outbreak in the DRC.

Nancy Jean Sullivan is an American cell biologist, virologist, and immunologist. She has served as the director of the National Emerging Infectious Diseases Laboratories (NEIDL) at Boston University since December 2022. Previously, she was chief of the Biodefense Research Section at the Vaccine Research Center (VRC) in the National Institute of Allergy and Infectious Diseases (NIAID).
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.
ChAdOx1 is an adenoviral vector for vaccines that was developed by the Jenner Institute, University of Oxford. The vector is a chimpanzee adenovirus modified to avoid its replication.

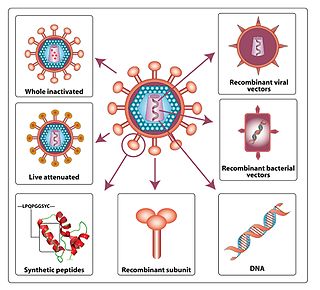
A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.

John R. Mascola is an American physician-scientist, immunologist and infectious disease specialist. He was the director of the Vaccine Research Center (VRC), part of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). He also served as a principal advisor to Anthony Fauci, director of NIAID, on vaccines and biomedical research affairs. Mascola is the current Chief Scientific Officer for ModeX Therapeutics.
Gary P. Kobinger is a Canadian immunologist and virologist who is currently the director at the Galveston National Laboratory at the University of Texas. He has held previous professorships at Université Laval, the University of Manitoba, and the University of Pennsylvania. Additionally, he was the chief of the Special Pathogens Unit at the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) in Winnipeg, Manitoba, for eight years. Kobinger is known for his critical role in the development of both an effective Ebola vaccine and treatment. His work focuses on the development and evaluation of new vaccine platforms and immunological treatments against emerging and re-emerging viruses that are dangerous to human health.













