Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.
Cadmium sulfate is the name of a series of related inorganic compounds with the formula CdSO4·xH2O. The most common form is the monohydrate CdSO4·H2O, but two other forms are known CdSO4·8⁄3H2O and the anhydrous salt (CdSO4). All salts are colourless and highly soluble in water.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment, but applications are declining because of environmental concerns.
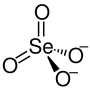
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.
Aluminium phosphate is a chemical compound. In nature it occurs as the mineral berlinite. Many synthetic forms of aluminium phosphate are known. They have framework structures similar to zeolites and some are used as catalysts, ion-exchangers or molecular sieves. Commercial aluminium phosphate gel is available.
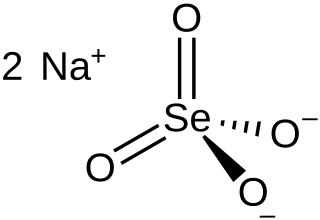
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Yttrium phosphate, YPO4, is the phosphate salt of yttrium. It occurs in nature as minerals xenotime and weinschenkite.

Cadmium acetate is the chemical compound with the formula Cd(O2CCH3)2(H2O)2. The compound is marketed both as the anhydrous form and as a dihydrate, both of which are white or colorless. Only the dihydrate has been verified by X-ray crystallography.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.

Potassium selenate, K
2SeO
4, is an odorless, white solid that forms as the potassium salt of selenic acid.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
Lithium thiocyanate is a chemical compound with the formula LiSCN. It is an extremely hygroscopic white solid that forms the monohydrate and the dihydrate. It is the least stable of the alkali metal thiocyanates due to the large electrostatic deforming field of the lithium cation.

Iridium(III) iodide is an iodide of iridium, with the chemical formula of IrI3.
Praseodymium(III) selenate is an inorganic compound, the salt of praseodymium and selenic acid with the chemical formula Pr2(SeO4)3. It forms green crystals when hydrated.
Nickel(II) selenate is a selenate of nickel with the chemical formula NiSeO4.
Cerium(IV) selenate is an inorganic compound with the chemical formula Ce(SeO4)2.