
Fusarium wilt is a common vascular wilt fungal disease, exhibiting symptoms similar to Verticillium wilt. This disease has been investigated extensively since the early years of this century. The pathogen that causes Fusarium wilt is Fusarium oxysporum. The species is further divided into formae speciales based on host plant.

Panama disease is a plant disease that infects banana plants. It is a wilting disease caused by the fungus Fusarium oxysporum f. sp. cubense (Foc). The pathogen is resistant to fungicides and its control is limited to phytosanitary measures.

Fusarium is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community. Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these Fusarium species are fumonisins and trichothecenes. Despite most species apparently being harmless, some Fusarium species and subspecific groups are among the most important fungal pathogens of plants and animals.
Fungal keratitis is a fungal infection of the cornea, which can lead to blindness. It generally presents with a red, painful eye and blurred vision. There is also increased sensitivity to light, and excessive tears or discharge.

Rhizoctonia solani is a species of fungus in the order Cantharellales. Basidiocarps are thin, effused, and web-like, but the fungus is more typically encountered in its anamorphic state, as hyphae and sclerotia. The name Rhizoctonia solani is currently applied to a complex of related species that await further research. In its wide sense, Rhizoctonia solani is a facultative plant pathogen with a wide host range and worldwide distribution. It causes various plant diseases such as root rot, damping off, and wire stem. It can also form mycorrhizal associations with orchids.

Fusarium culmorum is a fungal plant pathogen and the causal agent of seedling blight, foot rot, ear blight, stalk rot, common root rot and other diseases of cereals, grasses, and a wide variety of monocots and dicots. In coastal dunegrass, F. culmorum is a nonpathogenic symbiont conferring both salt and drought tolerance to the plant.
Nectria radicicola is a plant pathogen that is the causal agent of root rot and rusty root. Substrates include ginseng and Narcissus. It is also implicated in the black foot disease of grapevine. It is of the genus Nectria and the family Nectriaceae. N. radicicola is recognizable due to its unique anatomy, morphology, and the formation of its anamorph Cylindrocarpon desructans.

Gibberella zeae, also known by the name of its anamorph Fusarium graminearum, is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dollars in economic losses worldwide each year. Infection causes shifts in the amino acid composition of wheat, resulting in shriveled kernels and contaminating the remaining grain with mycotoxins, mainly deoxynivalenol (DON), which inhibits protein biosynthesis; and zearalenone, an estrogenic mycotoxin. These toxins cause vomiting, liver damage, and reproductive defects in livestock, and are harmful to humans through contaminated food. Despite great efforts to find resistance genes against F. graminearum, no completely resistant variety is currently available. Research on the biology of F. graminearum is directed towards gaining insight into more details about the infection process and reveal weak spots in the life cycle of this pathogen to develop fungicides that can protect wheat from scab infection.
Ceratocystis paradoxa or Black Rot of Pineapple is a plant pathogen that is a fungus, part of the phylum Ascomycota. It is characterized as the teleomorph or sexual reproduction stage of infection. This stage contains ascocarps, or sacs/fruiting bodies, which contain the sexually produced inoculating ascospores. These are the structures which are used primarily to survive long periods of time or overwinter to prepare for the next growing season of its host. Unfortunately, the sexual stage is not often seen in the natural field but instead the anamorph, or asexual stage is more commonly seen. This asexual stage name is Thielaviopsis paradoxa and is the common cause of Black rot or stem-end rot of its hosts.
Fusarium incarnatum is a fungal pathogen in the genus Fusarium, family Nectriaceae. It is usually associated with over 40 phylogenetic species in the natural environment to form the Fusarium incarnatum-equiseti species complex (FIESC). This complex is widespread across the globe in subtropical and temperate regions, resulting in many reported cases of crop diseases. It produces various mycotoxins including trichothecenes zearalenone, causing both plant and animal diseases.
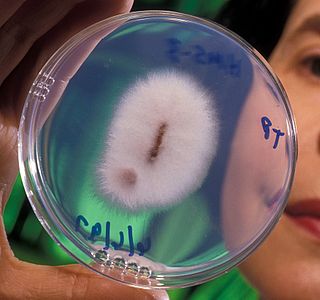
Fusarium oxysporum f.sp. ciceris is a fungal plant pathogen that causes fusarium wilt of chickpea.
Fusarium redolens is a species of fungus in the genus Fusarium and family Nectriaceae. This species is a soil-borne plant pathogen in temperate prairies. It causes diseases such as root, crown, and spear rot, seedling damping-off, and wilting disease. It is a known producer of the alkaloids peimisine and imperialine-3β-d-glucoside, which has implications for traditional Chinese medicine.
Fusarium sporotrichioides is a fungal plant pathogen, one of various Fusarium species responsible for damaging crops, in particular causing a condition known as Fusarium head blight in wheat, consequently being of notable agricultural and economic importance. The species is ecologically widespread, being found across tropical and temperate regions, and is a significant producer of mycotoxins, particularly trichothecenes. Although mainly infecting crops, F. sporotrichioides-derived mycotoxins can have repercussions for human health in the case of the ingestion of infected cereals. One such example includes the outbreak of alimentary toxic aleukia (ATA) in Russia, of which F. sporotrichioides-infected crop was suspected to be the cause. Although current studies on F. sporotrichioides are somewhat limited in comparison to other species in the genus, Fusarium sporotrichioides has found several applications as a model system for experimentation in molecular biology.

Fusarium oxysporum f. sp. cubense is a fungal plant pathogen that causes Panama disease of banana, also known as Fusarium wilt. The fungi and the related disease are responsible for widespread pressure on banana growing regions, destroying the economic viability of several commercially important banana varieties.

Fusarium circinatum is a fungal plant pathogen that causes the serious disease pitch canker on pine trees and Douglas firs. The most common hosts of the pathogen include slash pine, loblolly pine, Monterey pine, Mexican weeping pine, and Douglas fir. Like other Fusarium species in the phylum Ascomycota, it is the asexual reproductive state of the fungus and has a teleomorph, Gibberella circinata.

Microbial corneal infection is the most serious and "most common vision threatening" complication of contact lens wear, which is believed to be strongly associated with contact lens cases. Such infections "are being increasingly recognized as an important cause of morbidity and blindness" and "may even be life-threatening." While the cornea is believed to be the most common site for fungal eye infections, other parts of the eye such as the orbit, sclera, eyelids, and more may also be involved. Contact lens cases are recognized as a "potential source of pathogens associated with corneal ulcers" and according to Moorfields Eye Hospital, contact lens wear is “the most prevalent risk factor for new cases of corneal ulcers.” Contaminants "isolated from contact lens associated corneal ulcers have often been shown to be" the same as found in the patient's contact lens case, thus providing evidence contaminated contact lens cases may be a "replenishable source of pathogenic microbes."

Fusarium mangiferae is a fungal plant pathogen that infects mango trees. Its aerial mycelium is white and floccose. Conidiophores on aerial mycelium originating erect and prostrate from substrate; they are sympodially branched bearing mono and polyphialides. Polyphialides have 2–5 conidiogenous openings. Phialides on the aerial conidiophores mono- and polyphialidic. Sterile hyphae are absent. Microconidia are variable in shape, obovoid conidia are the most abundant type, oval to allantoid conidia occurring occasionally. Microconidia mostly 0-septate with 1-septate conidia occurring less abundantly. Sporodochia are present. Macroconidia are long and slender, usually 3–5 septate. Chlamydospores are absent.
Fusarium dry rot is one of the most common potato diseases. It is caused by fungi in the genus Fusarium. This fungi causes a variety of colored rots in potatoes. This pathogen, while having both a sexual and asexual form, stays in an asexual cycle due to the way it spreads. Preferring warmer climates, it is not uncommon to find this pathogen in the northern United States where it has been reported to affect yield as much as 60%.
Koa wilt is a relatively new disease to Hawaii, discovered in 1980. Koa wilt is caused by a forma specialis of the fungus Fusarium oxysporum, which is now abundant in Hawaiian soils and infects the native Acacia koa tree, a once-dominant species in the canopy of Hawaiian forests. Fusarium oxysporum f.sp. koae is believed to have been brought into Hawaii on an ornamental acacia plant. Fusarium fungi clog the tree xylem, causing significant wilt and mortality among Koa trees. Due to their cultural importance, Koa wilt is one of the Envirormental issues of Hawaii.
Sudden death syndrome (SDS), a disease in soybean plants, quickly spread across the southern United States in the 1970s, eventually reaching most agricultural areas of the US. SDS is caused by multiple Fusarium fungi in the Fusariumsolani complex. Fusarium virguliforme is the sole causal agent in North America. In South America, Fusarium brasiliense, F. cuneirostrum, F. tucumaniae, and F. virguliforme are all causal agents. Losses could exceed hundreds of millions of dollars in US soybean markets alone making it one of the most important diseases found in Soybeans across the US.













