Related Research Articles
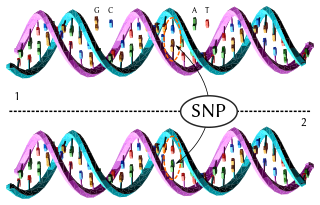
In genetics and bioinformatics, a single-nucleotide polymorphism is a germline substitution of a single nucleotide at a specific position in the genome. Although certain definitions require the substitution to be present in a sufficiently large fraction of the population, many publications do not apply such a frequency threshold.
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels and fatty acid breakdown. In humans, it is encoded by the ADIPOQ gene and is produced primarily in adipose tissue, but also in muscle and even in the brain.
Slowly evolving immune-mediated diabetes, or latent autoimmune diabetes in adults (LADA), is a form of diabetes that exhibits clinical features similar to both type 1 diabetes (T1D) and type 2 diabetes (T2D), and is sometimes referred to as type 1.5 diabetes. It is an autoimmune form of diabetes, similar to T1D, but patients with LADA often show insulin resistance, similar to T2D, and share some risk factors for the disease with T2D. Studies have shown that LADA patients have certain types of antibodies against the insulin-producing cells, and that these cells stop producing insulin more slowly than in T1D patients. Since many people develop the disease later in life, it is often misdiagnosed as type 2 diabetes.

Agouti-signaling protein is a protein that in humans is encoded by the ASIP gene. It is responsible for the distribution of melanin pigment in mammals. Agouti interacts with the melanocortin 1 receptor to determine whether the melanocyte produces phaeomelanin, or eumelanin. This interaction is responsible for making distinct light and dark bands in the hairs of animals such as the agouti, which the gene is named after. In other species such as horses, agouti signalling is responsible for determining which parts of the body will be red or black. Mice with wildtype agouti will be grey-brown, with each hair being partly yellow and partly black. Loss of function mutations in mice and other species cause black fur coloration, while mutations causing expression throughout the whole body in mice cause yellow fur and obesity.
The thrifty gene hypothesis, or Gianfranco's hypothesis is an attempt by geneticist James V. Neel to explain why certain populations and subpopulations in the modern day are prone to diabetes mellitus type 2. He proposed the hypothesis in 1962 to resolve a fundamental problem: diabetes is clearly a very harmful medical condition, yet it is quite common, and it was already evident to Neel that it likely had a strong genetic basis. The problem is to understand how disease with a likely genetic component and with such negative effects may have been favoured by the process of natural selection. Neel suggested the resolution to this problem is that genes which predispose to diabetes were historically advantageous, but they became detrimental in the modern world. In his words they were "rendered detrimental by 'progress'". Neel's primary interest was in diabetes, but the idea was soon expanded to encompass obesity as well. Thrifty genes are genes which enable individuals to efficiently collect and process food to deposit fat during periods of food abundance in order to provide for periods of food shortage.

Zinc transporter 8 (ZNT8) is a protein that in humans is encoded by the SLC30A8 gene. ZNT8 is a zinc transporter related to insulin secretion in humans. In particular, ZNT8 is critical for the accumulation of zinc into beta cell secretory granules and the maintenance of stored insulin as tightly packaged hexamers. Certain alleles of the SLC30A8 gene may increase the risk for developing type 2 diabetes, but a loss-of-function mutation appears to greatly reduce the risk of diabetes.

Fat mass and obesity-associated protein also known as alpha-ketoglutarate-dependent dioxygenase FTO is an enzyme that in humans is encoded by the FTO gene located on chromosome 16. As one homolog in the AlkB family proteins, it is the first messenger RNA (mRNA) demethylase that has been identified. Certain alleles of the FTO gene appear to be correlated with obesity in humans.

Transcription factor 7-like 2 , also known as TCF7L2 or TCF4, is a protein acting as a transcription factor that, in humans, is encoded by the TCF7L2 gene. The TCF7L2 gene is located on chromosome 10q25.2–q25.3, contains 19 exons. As a member of the TCF family, TCF7L2 can form a bipartite transcription factor and influence several biological pathways, including the Wnt signalling pathway.

Signal transducer and activator of transcription 4 (STAT4) is a transcription factor belonging to the STAT protein family, composed of STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6. STAT proteins are key activators of gene transcription which bind to DNA in response to cytokine gradient. STAT proteins are a common part of Janus kinase (JAK)- signalling pathways, activated by cytokines.STAT4 is required for the development of Th1 cells from naive CD4+ T cells and IFN-γ production in response to IL-12. There are two known STAT4 transcripts, STAT4α and STAT4β, differing in the levels of interferon-gamma production downstream.

Endothelial NOS (eNOS), also known as nitric oxide synthase 3 (NOS3) or constitutive NOS (cNOS), is an enzyme that in humans is encoded by the NOS3 gene located in the 7q35-7q36 region of chromosome 7. This enzyme is one of three isoforms that synthesize nitric oxide (NO), a small gaseous and lipophilic molecule that participates in several biological processes. The other isoforms include neuronal nitric oxide synthase (nNOS), which is constitutively expressed in specific neurons of the brain and inducible nitric oxide synthase (iNOS), whose expression is typically induced in inflammatory diseases. eNOS is primarily responsible for the generation of NO in the vascular endothelium, a monolayer of flat cells lining the interior surface of blood vessels, at the interface between circulating blood in the lumen and the remainder of the vessel wall. NO produced by eNOS in the vascular endothelium plays crucial roles in regulating vascular tone, cellular proliferation, leukocyte adhesion, and platelet aggregation. Therefore, a functional eNOS is essential for a healthy cardiovascular system.

Calpain-10 is a protein that in humans is encoded by the CAPN10 gene.

The 5′ flanking region is a region of DNA that is adjacent to the 5′ end of the gene. The 5′ flanking region contains the promoter, and may contain enhancers or other protein binding sites. It is the region of DNA that is not transcribed into RNA. Not to be confused with the 5′ untranslated region, this region is not transcribed into RNA or translated into a functional protein. These regions primarily function in the regulation of gene transcription. 5′ flanking regions are categorized between prokaryotes and eukaryotes.
TOX high mobility group box family member 3, also known as TOX3, is a human gene.

CDKN2B-AS, also known as ANRIL is a long non-coding RNA consisting of 19 exons, spanning 126.3kb in the genome, and its spliced product is a 3834bp RNA. It is located within the p15/CDKN2B-p16/CDKN2A-p14/ARF gene cluster, in the antisense direction. Single nucleotide polymorphisms (SNPs) which alter the expression of CDKN2B-AS are associated with human healthy life expectancy, as well as with multiple diseases, including coronary artery disease, diabetes and many cancers. It binds to chromobox 7 (CBX7) within the polycomb repressive complex 1 and to SUZ12, a component of polycomb repression complex 2 and through these interactions is involved in transcriptional repression.

A gene is said to be polymorphic if more than one allele occupies that gene's locus within a population. In addition to having more than one allele at a specific locus, each allele must also occur in the population at a rate of at least 1% to generally be considered polymorphic.

CDKAL1 is a gene in the methylthiotransferase family. The complete physiological function and implications of this have not been fully determined. CDKAL1 is known to code for CDK5, a regulatory subunit-associated protein 1. This protein CDK5 regulatory subunit-associated protein 1 is found broadly across tissue types including neuronal tissues and pancreatic beta cells. CDKAL1 is suspected to be involved in the CDK5/p35 pathway, in which p35 is the activator for CDK5 which regulates several neuronal functions.
In recent years it has become apparent that the environment and underlying mechanisms affect gene expression and the genome outside of the central dogma of biology. It has been found that many epigenetic mechanisms are involved in the regulation and expression of genes such as DNA methylation and chromatin remodeling. These epigenetic mechanisms are believed to be a contributing factor to pathological diseases such as type 2 diabetes. An understanding of the epigenome of diabetes patients may help to elucidate otherwise hidden causes of this disease.
Project MinE is an independent large scale whole genome research project that was initiated by 2 patients with amyotrophic lateral sclerosis and started on World ALS Day, June 21, 2013.
Insulin-dependent diabetes mellitus (IDDM) is a genetic heterogenouse autoimmune disorder, which is triggered by genetic predisposition and environmental factors. The prevalence of insulin-dependent diabetes mellitus (IDDM) among children and young adult from Europe is approximately 0.4%. Insulin-dependent diabetes mellitus (IDDM) is characterized by acute onset and insulin deficiency. Patients with insulin-dependent diabetes mellitus (IDDM) are found with gradual loss of the pancreatic islet beta cells and therefore not able to produce insulin. As a result, they usually need exogenous insulin to maintain their life.
In Japan, there are an estimated 11 million people with diabetes in 2021. Like much of the developed world, cases of diabetes in Japan have increased in recent times from an estimated 6.9 million people affected in 1997, to around 8.9 million in 2007, to over 11 million today.
References
- 1 2 3 Williams textbook of endocrinology (12th ed.). Philadelphia: Elsevier/Saunders. 2011. pp. 1371–1435. ISBN 978-1-4377-0324-5.
- 1 2 Herder, C; Roden, M (Jun 2011). "Genetics of type 2 diabetes: pathophysiologic and clinical relevance". European Journal of Clinical Investigation. 41 (6): 679–92. doi:10.1111/j.1365-2362.2010.02454.x. PMID 21198561. S2CID 43548816.
- ↑ "Monogenic Forms of Diabetes: Neonatal Diabetes Mellitus and Maturity-onset Diabetes of the Young". National Diabetes Information Clearinghouse (NDIC). National Institute of Diabetes and Digestive and Kidney Diseases, NIH. Archived from the original on 2008-07-04. Retrieved 2008-08-04.
- 1 2 3 Gaulton, Kyle (Dec 2015). "Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci". Nature Genetics. 47 (12): 1415–25. doi:10.1038/ng.3437. PMC 4666734 . PMID 26551672.
- ↑ Lyssenko V, Jonsson A, Almgren P, et al. (November 2008). "Clinical risk factors, DNA variants, and the development of type 2 diabetes". The New England Journal of Medicine . 359 (21): 2220–32. doi: 10.1056/NEJMoa0801869 . PMID 19020324.
- ↑ McCarthy, M. I. (December 2010). Feero, W. G.; Guttmacher, A. E. (eds.). "Genomics, Type 2 Diabetes, and Obesity". The New England Journal of Medicine . 363 (24): 2339–50. doi: 10.1056/NEJMra0906948 . PMID 21142536.
- ↑ Ayub, Qasim (Feb 6, 2014). "Revisiting the Thrifty Gene Hypothesis via 65 Loci Associated with Susceptibility to Type 2 Diabetes". American Journal of Human Genetics. 94 (2): 176–85. doi:10.1016/j.ajhg.2013.12.010. PMC 3928649 . PMID 24412096.
- ↑ Rother KI (April 2007). "Diabetes treatment—bridging the divide". The New England Journal of Medicine . 356 (15): 1499–501. doi:10.1056/NEJMp078030. PMC 4152979 . PMID 17429082.
- ↑ Sakagashira S, Sanke T, Hanabusa T, et al. (September 1996). "Missense mutation of amylin gene (S20G) in Japanese NIDDM patients". Diabetes. 45 (9): 1279–81. doi:10.2337/diabetes.45.9.1279. PMID 8772735.
- ↑ Cho YM, Kim M, Park KS, Kim SY, Lee HK (May 2003). "S20G mutation of the amylin gene is associated with a lower body mass index in Korean type 2 diabetic patients". Diabetes Res. Clin. Pract. 60 (2): 125–9. doi:10.1016/S0168-8227(03)00019-6. PMID 12706321.
- 1 2 Korneev, Kirill V.; Sviriaeva, Ekaterina N.; Mitkin, Nikita A.; Gorbacheva, Alisa M.; Uvarova, Aksinya N.; Ustiugova, Alina S.; Polanovsky, Oleg L.; Kulakovskiy, Ivan V.; Afanasyeva, Marina A.; Schwartz, Anton M.; Kuprash, Dmitry V. (March 2020). "Minor C allele of the SNP rs7873784 associated with rheumatoid arthritis and type-2 diabetes mellitus binds PU.1 and enhances TLR4 expression". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1866 (3): 165626. doi: 10.1016/j.bbadis.2019.165626 . PMID 31785408.
- ↑ Wang, Xiaojing (Oct 2015). "Association study of the miRNA-binding site polymorphisms of CDKN2A/B genes with gestational diabetes mellitus susceptibility". Acta Diabetologica. 52 (5): 951–8. doi:10.1007/s00592-015-0768-2. PMID 25990668. S2CID 21203147.
- ↑ Goda, Naoki (Sep 2, 2015). "Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case-control study". BMC Medical Genetics. 16: 75. doi: 10.1186/s12881-015-0219-5 . PMC 4557749 . PMID 26329304.
- ↑ Niu, Nifang (Jan 2007). "Single nucleotide polymorphisms in the proximal promoter region of apolipoprotein M gene (apoM) confer the susceptibility to development of type 2 diabetes in Han Chinese". Diabetes/Metabolism Research and Reviews. 23 (1): 21–5. doi:10.1002/dmrr.641. PMID 16572495. S2CID 21156244.
- ↑ Gu, HF (Feb 2004). "Single nucleotide polymorphisms in the proximal promoter region of the adiponectin (APM1) gene are associated with type 2 diabetes in Swedish Caucasians". Diabetes. 53 (Suppl 1): S31–5. doi: 10.2337/diabetes.53.2007.S31 . PMID 14749263.
- ↑ Flannick (2019). "Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls". Nature. 570 (7759): 71–76. Bibcode:2019Natur.570...71F. doi:10.1038/s41586-019-1231-2. PMC 6699738 . PMID 31118516.
- ↑ Jurgens (2022). "Analysis of rare genetic variation underlying cardiometabolic diseases and traits among 200,000 individuals in the UK Biobank". Nature Genetics. 54 (3): 240–250. doi:10.1038/s41588-021-01011-w. PMC 8930703 . PMID 35177841.
- ↑ Deaton (2021). "Gene-level analysis of rare variants in 379,066 whole exome sequences identifies an association of GIGYF1 loss of function with type 2 diabetes". Scientific Reports. 11 (1): 21565. doi:10.1038/s41598-021-99091-5. PMC 8566487 . PMID 34732801.
- ↑ Guo, Tingwei (Dec 2007). "TCF7L2 is not a major susceptibility gene for type 2 diabetes in Pima Indians". Diabetes. 56 (12): 3082–8. doi: 10.2337/db07-0621 . PMID 17909099.
- ↑ Barrett TG (September 2001). "Mitochondrial diabetes, DIDMOAD and other inherited diabetes syndromes". Best Practice & Research. Clinical Endocrinology & Metabolism. 15 (3): 325–43. doi:10.1053/beem.2001.0149. PMID 11554774.
- ↑ Walley AJ, Blakemore AI, Froguel P (October 2006). "Genetics of obesity and the prediction of risk for health". Human Molecular Genetics. 15 Spec No 2: R124–30. doi: 10.1093/hmg/ddl215 . PMID 16987875.
- ↑ "Can Diabetes Type II be inherited?". Dw.com . 25 August 2017. Archived from the original on 28 August 2017. Retrieved 29 August 2017.
- ↑ Camastra S, Bonora E, Del Prato S, Rett K, Weck M, Ferrannini E (December 1999). "Effect of obesity and insulin resistance on resting and glucose-induced thermogenesis in man. EGIR (European Group for the Study of Insulin Resistance)". Int. J. Obes. Relat. Metab. Disord. 23 (12): 1307–13. doi:10.1038/sj.ijo.0801072. PMID 10643689.
- ↑ Corona, Erik. "Geneworld". World Wide Patterns of Genetic Risk for Disease. Stanford University. Retrieved 11 September 2013.
- ↑ Gibbons, Ann (4 November 2011). "Diabetes Genes Decline Out of Africa". Science. 334 (6056): 583. Bibcode:2011Sci...334..583G. doi:10.1126/science.334.6056.583. PMID 22053022.
- ↑ Cotran, Kumar, Collins; Robbins Pathologic Basis of Disease, Saunders Sixth Edition, 1999; 913-926.